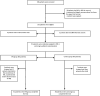
A Randomized Clinical Trial of the Efficacy and Safety of Interferon β-1a in Treatment of Severe COVID-19
- PMID: 32661006
- PMCID: PMC7449227
- DOI: 10.1128/AAC.01061-20
A Randomized Clinical Trial of the Efficacy and Safety of Interferon β-1a in Treatment of Severe COVID-19
Abstract
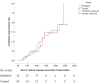
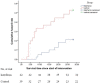
To the best of our knowledge, there is no published study on the use of interferon β-1a (IFN β-1a) in the treatment of severe COVID-19. In this randomized clinical trial, the efficacy and safety of IFN β-1a were evaluated in patients with severe COVID-19. Forty-two patients in the interferon group received IFN β-1a in addition to the national protocol medications (hydroxychloroquine plus lopinavir-ritonavir or atazanavir-ritonavir). Each 44-μg/ml (12 million IU/ml) dose of interferon β-1a was subcutaneously injected three times weekly for two consecutive weeks. The control group consisted of 39 patients who received only the national protocol medications. The primary outcome of the study was time to reach clinical response. Secondary outcomes were duration of hospital stay, length of intensive care unit stay, 28-day mortality, effect of early or late administration of IFN on mortality, adverse effects, and complications during the hospitalization. Between 29 February and 3 April 2020, 92 patients were recruited, and a total of 42 patients in the IFN group and 39 patients in the control group completed the study. As the primary outcome, time to the clinical response was not significantly different between the IFN and the control groups (9.7 ± 5.8 versus 8.3 ± 4.9 days, respectively, P = 0.95). On day 14, 66.7% versus 43.6% of patients in the IFN group and the control group, respectively, were discharged (odds ratio [OR], 2.5; 95% confidence interval [CI], 1.05 to 6.37). The 28-day overall mortality was significantly lower in the IFN than the control group (19% versus 43.6%, respectively, P = 0.015). Early administration significantly reduced mortality (OR, 13.5; 95% CI, 1.5 to 118). Although IFN did not change the time to reach the clinical response, adding it to the national protocol significantly increased discharge rate on day 14 and decreased 28-day mortality. (This study is in the Iranian Registry of Clinical Trials under identifier IRCT20100228003449N28.).
Keywords: COVID-19; clinical response; interferon; mortality.
Copyright © 2020 American Society for Microbiology.
Figures
Comment in
-
Reply to Dorgham et al., "Considering Personalized Interferon Beta Therapy for COVID-19".Antimicrob Agents Chemother. 2021 Mar 18;65(4):e00083-21. doi: 10.1128/AAC.00083-21. Print 2021 Mar 18. Antimicrob Agents Chemother. 2021. PMID: 33558288 Free PMC article. No abstract available.
-
Considering Personalized Interferon Beta Therapy for COVID-19.Antimicrob Agents Chemother. 2021 Mar 18;65(4):e00065-21. doi: 10.1128/AAC.00065-21. Print 2021 Mar 18. Antimicrob Agents Chemother. 2021. PMID: 33558300 Free PMC article. No abstract available.
Similar articles
-
A Retrospective Controlled Cohort Study of the Impact of Glucocorticoid Treatment in SARS-CoV-2 Infection Mortality.Antimicrob Agents Chemother. 2020 Aug 20;64(9):e01168-20. doi: 10.1128/AAC.01168-20. Print 2020 Aug 20. Antimicrob Agents Chemother. 2020. PMID: 32571831 Free PMC article.
-
Effectiveness of Interferon Beta 1a, compared to Interferon Beta 1b and the usual therapeutic regimen to treat adults with moderate to severe COVID-19: structured summary of a study protocol for a randomized controlled trial.Trials. 2020 Jun 3;21(1):473. doi: 10.1186/s13063-020-04382-3. Trials. 2020. PMID: 32493468 Free PMC article.
-
Interferon β-1b in treatment of severe COVID-19: A randomized clinical trial.Int Immunopharmacol. 2020 Nov;88:106903. doi: 10.1016/j.intimp.2020.106903. Epub 2020 Aug 24. Int Immunopharmacol. 2020. PMID: 32862111 Free PMC article. Clinical Trial.
-
Clinical Trials of Repurposed Antivirals for SARS-CoV-2.Antimicrob Agents Chemother. 2020 Aug 20;64(9):e01101-20. doi: 10.1128/AAC.01101-20. Print 2020 Aug 20. Antimicrob Agents Chemother. 2020. PMID: 32631826 Free PMC article. Review.
-
Umifenovir in hospitalized moderate to severe COVID-19 patients: A randomized clinical trial.Int Immunopharmacol. 2021 Oct;99:107969. doi: 10.1016/j.intimp.2021.107969. Epub 2021 Jul 10. Int Immunopharmacol. 2021. PMID: 34273635 Free PMC article. Review.
Cited by
-
Potential limitations in systematic review studies assessing the effect of the main intervention for treatment/therapy of COVID-19 patients: An overview.Front Med (Lausanne). 2022 Sep 15;9:966632. doi: 10.3389/fmed.2022.966632. eCollection 2022. Front Med (Lausanne). 2022. PMID: 36203750 Free PMC article.
-
Harnessing Type I IFN Immunity Against SARS-CoV-2 with Early Administration of IFN-β.J Clin Immunol. 2021 Oct;41(7):1425-1442. doi: 10.1007/s10875-021-01068-6. Epub 2021 Jun 8. J Clin Immunol. 2021. PMID: 34101091 Free PMC article. No abstract available.
-
Blood Interferon-α Levels and Severity, Outcomes, and Inflammatory Profiles in Hospitalized COVID-19 Patients.Front Immunol. 2021 Mar 9;12:648004. doi: 10.3389/fimmu.2021.648004. eCollection 2021. Front Immunol. 2021. PMID: 33767713 Free PMC article.
-
Biologics in COVID-19 So Far: Systematic Review.Pharmaceuticals (Basel). 2022 Jun 23;15(7):783. doi: 10.3390/ph15070783. Pharmaceuticals (Basel). 2022. PMID: 35890081 Free PMC article. Review.
-
Coronavirus Disease (COVID-19) Control between Drug Repurposing and Vaccination: A Comprehensive Overview.Vaccines (Basel). 2021 Nov 12;9(11):1317. doi: 10.3390/vaccines9111317. Vaccines (Basel). 2021. PMID: 34835248 Free PMC article. Review.
References
-
- Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. 2020. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395:1054–1062. doi:10.1016/S0140-6736(20)30566-3. - DOI - PMC - PubMed
-
- Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Zhou X, Du C, Zhang Y, Song J, Wang S, Chao Y, Yang Z, Xu J, Zhou X, Chen D, Xiong W, Xu L, Zhou F, Jiang J, Bai C, Zheng J, Song Y. 2020. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 180:e200994. doi:10.1001/jamainternmed.2020.0994. - DOI - PMC - PubMed
-
- World Health Organization. 2020. WHO coronavirus disease (COVID-19) dashboard. World Health Organization, Geneva, Switzerland: https://covid19.who.int/?gclid=Cj0KCQjwl4v4BRDaARIsAFjATPmRcsB7VPlJAxJMh.... Accessed 6 July 2020.
-
- Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, Li X, Xia J, Chen N, Xiang J, Yu T, Bai T, Xie X, Zhang L, Li C, Yuan Y, Chen H, Li H, Huang H, Tu S, Gong F, Liu Y, Wei Y, Dong C, Zhou F, Gu X, Xu J, Liu Z, Zhang Y, Li H, Shang L, Wang K, Li K, Zhou X, Dong X, Qu Z, Lu S, Hu X, Ruan S, Luo S, Wu J, Peng L, Cheng F, Pan L, Zou J, Jia C, Wang J, Liu X, Wang S, Wu X, Ge Q, He J, Zhan H, Qiu F, Guo L, Huang C, Jaki T, Hayden FG, Horby PW, Zhang D, Wang C. 2020. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med 382:1787–1799. doi:10.1056/NEJMoa2001282. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous