Acute murine cytomegalovirus disrupts established transplantation tolerance and causes recipient allo-sensitization
- PMID: 32659030
- PMCID: PMC7855505
- DOI: 10.1111/ajt.16197
Acute murine cytomegalovirus disrupts established transplantation tolerance and causes recipient allo-sensitization
Abstract
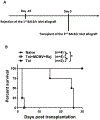
We have previously shown that acute cytomegalovirus (CMV) infection disrupts the induction of transplantation tolerance. However, what impact acute CMV infection would have on the maintenance of established tolerance and on subsequent recipient allo-sensitization is a clinically important unanswered question. Here we used an allogeneic murine islet transplantation tolerance model to examine the impact of acute CMV infection on: (a) disruption of established transplantation tolerance during tolerance maintenance; and (b) the possibility of recipient allo-sensitization by CMV-mediated disruption of stable tolerance. We demonstrated that acute CMV infection abrogated transplantation tolerance during the maintenance stage in 50%-60% recipients. We further demonstrated that acute CMV infection-mediated tolerance disruption led to recipient allo-sensitization by reverting the tolerant state of allo-specific T cells and promoting their differentiation to allo-specific memory cells. Consequently, a second same-donor islet allograft was rejected in an accelerated fashion by these recipients. Our study therefore supports close monitoring for allo-sensitization in previously tolerant transplant recipients in whom tolerance maintenance is disrupted by an episode of acute CMV infection.
Keywords: alloantibody; basic (laboratory) research/science; immunobiology; immunosuppression/immune modulation; infection and infectious agents - viral: Cytomegalovirus (CMV); islet transplantation; re-transplantation; rejection: T cell-mediated (TCMR); tolerance.
© 2020 The American Society of Transplantation and the American Society of Transplant Surgeons.
Figures
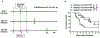
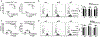
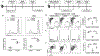

Similar articles
-
Murine cytomegalovirus dissemination but not reactivation in donor-positive/recipient-negative allogeneic kidney transplantation can be effectively prevented by transplant immune tolerance.Kidney Int. 2020 Jul;98(1):147-158. doi: 10.1016/j.kint.2020.01.034. Epub 2020 Feb 21. Kidney Int. 2020. PMID: 32471635 Free PMC article.
-
Rat Cytomegalovirus Vaccine Prevents Accelerated Chronic Rejection in CMV-Naïve Recipients of Infected Donor Allograft Hearts.Am J Transplant. 2015 Jul;15(7):1805-16. doi: 10.1111/ajt.13188. Epub 2015 Mar 12. Am J Transplant. 2015. PMID: 25766876 Free PMC article.
-
Murine CMV induces type 1 IFN that impairs differentiation of MDSCs critical for transplantation tolerance.Blood Adv. 2018 Mar 27;2(6):669-680. doi: 10.1182/bloodadvances.2017012187. Blood Adv. 2018. PMID: 29563123 Free PMC article.
-
The association of cytomegalovirus infection and cytomegalovirus serostatus with invasive fungal infections in allogeneic haematopoietic stem cell transplant recipients: a systematic review and meta-analysis.Clin Microbiol Infect. 2022 Mar;28(3):332-344. doi: 10.1016/j.cmi.2021.10.008. Epub 2021 Nov 6. Clin Microbiol Infect. 2022. PMID: 34752926 Review.
-
Optimizing the treatment of cytomegalovirus infection in allo-HSCT recipients.Expert Rev Clin Immunol. 2023 Feb;19(2):227-235. doi: 10.1080/1744666X.2023.2161510. Epub 2023 Jan 1. Expert Rev Clin Immunol. 2023. PMID: 36541485 Review.
Cited by
-
Negative Vaccination Strategies for Promotion of Transplant Tolerance.Transplantation. 2024 Aug 1;108(8):1715-1729. doi: 10.1097/TP.0000000000004911. Epub 2024 Feb 16. Transplantation. 2024. PMID: 38361234 Review.
-
Apoptotic Donor Cells in Transplantation.Front Immunol. 2021 Feb 25;12:626840. doi: 10.3389/fimmu.2021.626840. eCollection 2021. Front Immunol. 2021. PMID: 33717145 Free PMC article. Review.
-
Conversion of CD73hiFR4hi anergic T cells to IFN-γ-producing effector cells disrupts established immune tolerance.J Clin Invest. 2023 Mar 1;133(5):e163872. doi: 10.1172/JCI163872. J Clin Invest. 2023. PMID: 36649085 Free PMC article. No abstract available.
References
-
- Stehlik J, Kobashigawa J, Hunt SA, Reichenspurner H & Kirklin JK Honoring 50 Years of Clinical Heart Transplantation in Circulation: In-Depth State-of-the-Art Review. Circulation 137, 71–87 (2018). - PubMed
-
- Kawai T, et al. Long-term results in recipients of combined HLA-mismatched kidney and bone marrow transplantation without maintenance immunosuppression. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 14, 1599–1611 (2014). - PMC - PubMed
-
- Todo S, et al. A pilot study of operational tolerance with a regulatory T-cell-based cell therapy in living donor liver transplantation. Hepatology (Baltimore, Md.) 64, 632–643 (2016). - PubMed
-
- Oura T, et al. Long-term hepatic allograft acceptance based on CD40 blockade by ASKP1240 in nonhuman primates. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 12, 1740–1754 (2012). - PubMed

