Palmitoylated Proteins in Dendritic Spine Remodeling
- PMID: 32655390
- PMCID: PMC7325885
- DOI: 10.3389/fnsyn.2020.00022
Palmitoylated Proteins in Dendritic Spine Remodeling
Abstract
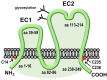
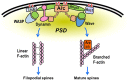
Activity-responsive changes in the actin cytoskeleton are required for the biogenesis, motility, and remodeling of dendritic spines. These changes are governed by proteins that regulate the polymerization, depolymerization, bundling, and branching of actin filaments. Thus, processes that have been extensively characterized in the context of non-neuronal cell shape change and migration are also critical for learning and memory. In this review article, we highlight actin regulatory proteins that associate, at least transiently, with the dendritic plasma membrane. All of these proteins have been shown, either in directed studies or in high-throughput screens, to undergo palmitoylation, a potentially reversible, and stimulus-dependent cysteine modification. Palmitoylation increases the affinity of peripheral proteins for the membrane bilayer and contributes to their subcellular localization and recruitment to cholesterol-rich membrane microdomains.
Keywords: actin assembly; cytoskeletal remodeling; dendritic spines; palmitoylation; synaptic plasticity.
Copyright © 2020 Albanesi, Barylko, DeMartino and Jameson.
Figures

Similar articles
-
Roles of palmitoylation in structural long-term synaptic plasticity.Mol Brain. 2021 Jan 11;14(1):8. doi: 10.1186/s13041-020-00717-y. Mol Brain. 2021. PMID: 33430908 Free PMC article. Review.
-
Stabilization of Spine Synaptopodin by mGluR1 Is Required for mGluR-LTD.J Neurosci. 2022 Mar 2;42(9):1666-1678. doi: 10.1523/JNEUROSCI.1466-21.2022. Epub 2022 Jan 19. J Neurosci. 2022. PMID: 35046120 Free PMC article.
-
Actin Tyrosine-53-Phosphorylation in Neuronal Maturation and Synaptic Plasticity.J Neurosci. 2016 May 11;36(19):5299-313. doi: 10.1523/JNEUROSCI.2649-15.2016. J Neurosci. 2016. PMID: 27170127 Free PMC article.
-
The role of the drebrin/EB3/Cdk5 pathway in dendritic spine plasticity, implications for Alzheimer's disease.Brain Res Bull. 2016 Sep;126(Pt 3):293-299. doi: 10.1016/j.brainresbull.2016.06.015. Epub 2016 Jun 27. Brain Res Bull. 2016. PMID: 27365229
-
Dendritic Spines in Alzheimer's Disease: How the Actin Cytoskeleton Contributes to Synaptic Failure.Int J Mol Sci. 2020 Jan 30;21(3):908. doi: 10.3390/ijms21030908. Int J Mol Sci. 2020. PMID: 32019166 Free PMC article. Review.
Cited by
-
Rho Signaling in Synaptic Plasticity, Memory, and Brain Disorders.Front Cell Dev Biol. 2021 Oct 4;9:729076. doi: 10.3389/fcell.2021.729076. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34671600 Free PMC article. Review.
-
Neurons and Astrocytes Elicit Brain Region Specific Transcriptional Responses to Prion Disease in the Murine CA1 and Thalamus.Front Neurosci. 2022 May 16;16:918811. doi: 10.3389/fnins.2022.918811. eCollection 2022. Front Neurosci. 2022. PMID: 35651626 Free PMC article.
-
S-Palmitoylation of Synaptic Proteins as a Novel Mechanism Underlying Sex-Dependent Differences in Neuronal Plasticity.Int J Mol Sci. 2021 Jun 10;22(12):6253. doi: 10.3390/ijms22126253. Int J Mol Sci. 2021. PMID: 34200797 Free PMC article.
-
Altered Protein Palmitoylation as Disease Mechanism in Neurodegenerative Disorders.J Neurosci. 2024 Oct 2;44(40):e1225242024. doi: 10.1523/JNEUROSCI.1225-24.2024. J Neurosci. 2024. PMID: 39358031 Review.
-
Mimicking Protein Kinase C Phosphorylation Inhibits Arc/Arg3.1 Palmitoylation and Its Interaction with Nucleic Acids.Int J Mol Sci. 2024 Jan 8;25(2):780. doi: 10.3390/ijms25020780. Int J Mol Sci. 2024. PMID: 38255853 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

