The precious fluorine on the ring: fluorine NMR for biological systems
- PMID: 32651751
- PMCID: PMC7539674
- DOI: 10.1007/s10858-020-00331-z
The precious fluorine on the ring: fluorine NMR for biological systems
Abstract
The fluorine-19 nucleus was recognized early to harbor exceptional properties for NMR spectroscopy. With 100% natural abundance, a high gyromagnetic ratio (83% sensitivity compared to 1H), a chemical shift that is extremely sensitive to its surroundings and near total absence in biological systems, it was destined to become a favored NMR probe, decorating small and large molecules. However, after early excitement, where uptake of fluorinated aromatic amino acids was explored in a series of animal studies, 19F-NMR lost popularity, especially in large molecular weight systems, due to chemical shift anisotropy (CSA) induced line broadening at high magnetic fields. Recently, two orthogonal approaches, (i) CF3 labeling and (ii) aromatic 19F-13C labeling leveraging the TROSY (Transverse Relaxation Optimized Spectroscopy) effect have been successfully applied to study large biomolecular systems. In this perspective, we will discuss the fascinating early work with fluorinated aromatic amino acids, which reveals the enormous potential of these non-natural amino acids in biological NMR and the potential of 19F-NMR to characterize protein and nucleic acid structure, function and dynamics in the light of recent developments. Finally, we explore how fluorine NMR might be exploited to implement small molecule or fragment screens that resemble physiological conditions and discuss the opportunity to follow the fate of small molecules in living cells.
Keywords: 4-fluorophenylalanine; Drug discovery; Fluorine NMR; Nucleic acids; Proteins; TROSY.
Conflict of interest statement
Figures
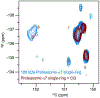
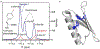
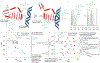
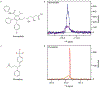
Similar articles
-
Aromatic 19F-13C TROSY: a background-free approach to probe biomolecular structure, function, and dynamics.Nat Methods. 2019 Apr;16(4):333-340. doi: 10.1038/s41592-019-0334-x. Epub 2019 Mar 11. Nat Methods. 2019. PMID: 30858598 Free PMC article.
-
2-Fluorotyrosine is a valuable but understudied amino acid for protein-observed 19F NMR.J Biomol NMR. 2020 Jan;74(1):61-69. doi: 10.1007/s10858-019-00290-0. Epub 2019 Nov 23. J Biomol NMR. 2020. PMID: 31760571
-
Small, but powerful and attractive: 19F in biomolecular NMR.Structure. 2022 Jan 6;30(1):6-14. doi: 10.1016/j.str.2021.09.009. Epub 2021 Dec 13. Structure. 2022. PMID: 34995480 Free PMC article. Review.
-
Fluorinated Tags to Study Protein Conformation and Interactions Using 19F NMR.Chembiochem. 2024 Aug 1;25(15):e202400195. doi: 10.1002/cbic.202400195. Epub 2024 Jun 24. Chembiochem. 2024. PMID: 38744671 Review.
-
Feasibility of trifluoromethyl TROSY NMR at high magnetic fields.J Biomol NMR. 2019 Nov;73(10-11):519-523. doi: 10.1007/s10858-019-00266-0. Epub 2019 Jul 2. J Biomol NMR. 2019. PMID: 31267350
Cited by
-
Modulating co-translational protein folding by rational design and ribosome engineering.Nat Commun. 2022 Jul 22;13(1):4243. doi: 10.1038/s41467-022-31906-z. Nat Commun. 2022. PMID: 35869078 Free PMC article.
-
19F NMR as a tool in chemical biology.Beilstein J Org Chem. 2021 Jan 28;17:293-318. doi: 10.3762/bjoc.17.28. eCollection 2021. Beilstein J Org Chem. 2021. PMID: 33564338 Free PMC article. Review.
-
Engineered Proteins and Materials Utilizing Residue-Specific Noncanonical Amino Acid Incorporation.Chem Rev. 2024 Aug 14;124(15):9113-9135. doi: 10.1021/acs.chemrev.3c00855. Epub 2024 Jul 15. Chem Rev. 2024. PMID: 39008623 Free PMC article. Review.
-
Decorating phenylalanine side-chains with triple labeled 13C/19F/2H isotope patterns.J Biomol NMR. 2024 Sep;78(3):139-147. doi: 10.1007/s10858-024-00440-z. Epub 2024 Mar 21. J Biomol NMR. 2024. PMID: 38509441 Free PMC article.
-
Cell-free synthesis of amyloid fibrils with infectious properties and amenable to sub-milligram magic-angle spinning NMR analysis.Commun Biol. 2022 Nov 9;5(1):1202. doi: 10.1038/s42003-022-04175-1. Commun Biol. 2022. PMID: 36352173 Free PMC article.
References
-
- Ye L, Van Eps N, Zimmer M, Ernst OP & Prosser RS Activation of the A2A adenosine G-protein-coupled receptor by conformational selection. Nature 533, 265–8 (2016). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

