Advances in Anti-Cancer Immunotherapy: Car-T Cell, Checkpoint Inhibitors, Dendritic Cell Vaccines, and Oncolytic Viruses, and Emerging Cellular and Molecular Targets
- PMID: 32645977
- PMCID: PMC7408985
- DOI: 10.3390/cancers12071826
Advances in Anti-Cancer Immunotherapy: Car-T Cell, Checkpoint Inhibitors, Dendritic Cell Vaccines, and Oncolytic Viruses, and Emerging Cellular and Molecular Targets
Abstract
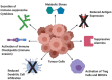
Unlike traditional cancer therapies, such as surgery, radiation and chemotherapy that are typically non-specific, cancer immunotherapy harnesses the high specificity of a patient's own immune system to selectively kill cancer cells. The immune system is the body's main cancer surveillance system, but cancers may evade destruction thanks to various immune-suppressing mechanisms. We therefore need to deploy various immunotherapy-based strategies to help bolster the anti-tumour immune responses. These include engineering T cells to express chimeric antigen receptors (CARs) to specifically recognise tumour neoantigens, inactivating immune checkpoints, oncolytic viruses and dendritic cell (DC) vaccines, which have all shown clinical benefit in certain cancers. However, treatment efficacy remains poor due to drug-induced adverse events and immunosuppressive tendencies of the tumour microenvironment. Recent preclinical studies have unveiled novel therapies such as anti-cathepsin antibodies, galectin-1 blockade and anti-OX40 agonistic antibodies, which may be utilised as adjuvant therapies to modulate the tumour microenvironment and permit more ferocious anti-tumour immune response.
Keywords: CAR-T cell; OX40; cathepsin D; checkpoint inhibitor; dendritic cell vaccines; drug resistance; galectin-1; immunosuppression; oncolytic viruses; tumour-induced immune evasion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Harnessing the Power of Onco-Immunotherapy with Checkpoint Inhibitors.Viruses. 2015 Nov 13;7(11):5889-901. doi: 10.3390/v7112914. Viruses. 2015. PMID: 26580645 Free PMC article. Review.
-
Oncolytic Newcastle disease virus expressing a checkpoint inhibitor as a radioenhancing agent for murine melanoma.EBioMedicine. 2019 Nov;49:96-105. doi: 10.1016/j.ebiom.2019.10.032. Epub 2019 Oct 29. EBioMedicine. 2019. PMID: 31676387 Free PMC article.
-
Recent updates on cancer immunotherapy.Precis Clin Med. 2018 Sep;1(2):65-74. doi: 10.1093/pcmedi/pby011. Epub 2018 Sep 6. Precis Clin Med. 2018. PMID: 30687562 Free PMC article. Review.
-
Chimeric Antigen Receptors for the Tumour Microenvironment.Adv Exp Med Biol. 2020;1263:117-143. doi: 10.1007/978-3-030-44518-8_8. Adv Exp Med Biol. 2020. PMID: 32588326 Review.
-
Viro-immune therapy: A new strategy for treatment of pancreatic cancer.World J Gastroenterol. 2016 Jan 14;22(2):748-63. doi: 10.3748/wjg.v22.i2.748. World J Gastroenterol. 2016. PMID: 26811622 Free PMC article. Review.
Cited by
-
Clinical significance of circulating tumor DNA in localized non-small cell lung cancer: a systematic review and meta-analysis.Clin Exp Med. 2023 Sep;23(5):1621-1631. doi: 10.1007/s10238-022-00924-y. Epub 2022 Oct 31. Clin Exp Med. 2023. PMID: 36315311
-
Mesenchymal stem cell carriers enhance anti-tumor efficacy of oncolytic virotherapy.Oncol Lett. 2021 Apr;21(4):238. doi: 10.3892/ol.2021.12499. Epub 2021 Jan 28. Oncol Lett. 2021. PMID: 33664802 Free PMC article. Review.
-
Patient-Derived Microphysiological Systems for Precision Medicine.Adv Healthc Mater. 2024 Mar;13(7):e2303161. doi: 10.1002/adhm.202303161. Epub 2023 Dec 10. Adv Healthc Mater. 2024. PMID: 38010253 Free PMC article. Review.
-
Cyclophosphamide augments the efficacy of in situ vaccination in a mouse melanoma model.Front Oncol. 2023 Sep 6;13:1200436. doi: 10.3389/fonc.2023.1200436. eCollection 2023. Front Oncol. 2023. PMID: 37746303 Free PMC article.
-
Novel therapeutic agents in clinical trials: emerging approaches in cancer therapy.Discov Oncol. 2024 Aug 11;15(1):342. doi: 10.1007/s12672-024-01195-7. Discov Oncol. 2024. PMID: 39127974 Free PMC article. Review.
References
-
- Cancer Research UK. [(accessed on 22 May 2020)]; Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics-f....
-
- Fiorica F., Trovo M., Ottaiano A., Nasti G., Carandina I., Marzola M., De Paoli P., Berretta M. Can the addition of radiotherapy postoperatively increase clinical outcome of patients with gastric cancer? A systematic review of the literature and meta-analysis. Oncotarget. 2018;9:10734–10744. doi: 10.18632/oncotarget.23754. - DOI - PMC - PubMed
-
- Ma Q., Gonzalo-Daganzo R.M., Junghans R.P. Genetically engineered T cells as adoptive immunotherapy of cancer. Cancer Chemother Biol. Response Modif. 2002;20:315–341. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

