Effects of pentosan polysulfate on cell proliferation, cell cycle progression and cyclin-dependent kinases expression in canine articular chondrocytes
- PMID: 32641601
- PMCID: PMC7468060
- DOI: 10.1292/jvms.20-0091
Effects of pentosan polysulfate on cell proliferation, cell cycle progression and cyclin-dependent kinases expression in canine articular chondrocytes
Abstract
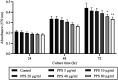
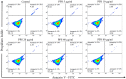
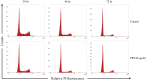
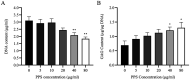
Pentosan polysulfate (PPS) is a semi-synthetic sulfated polysaccharide compound which has been shown the benefits on therapeutic treatment for osteoarthritis (OA) and has been proposed as a disease modifying osteoarthritis drugs (DMOADs). This study investigated the effects of PPS on cell proliferation, particularly in cell cycle modulation and phenotype promotion of canine articular chondrocytes (AC). Canine AC were treated with PPS (0-80 µg/ml) for 24, 48 and 72 hr. The effect of PPS on cell viability, cell proliferation and cell cycle distribution were analyzed by MTT assay, DNA quantification and flow cytometry. Chondrocyte phenotype was analyzed by quantitative real-time PCR (qPCR) and glycosaminoglycan (GAG) quantification. PPS significantly reduced AC proliferation through cell cycle modulation particularly by maintaining a significantly higher proportion of chondrocytes in the G1 phase and a significantly lower proportion in the S phase of the cell cycle in a concentration- and time-dependent manner. While the proportion of chondrocytes in G1 phase corresponded with the significant downregulation of cyclin-dependent kinase (CDK) 1 and 4. Furthermore, the study confirms that PPS promotes a chondrogenic phenotype of AC through significant upregulation of collagen type II (Col2A1) mRNA and GAG synthesis. The effect of PPS on the inhibition of chondrocyte proliferation while promoting a chondrocyte phenotype could be beneficial in the early stages of OA treatment, which transient increase in proliferative activity of chondrocytes with subsequent phenotypic shift and less productive in an essential component of extracellular matrix (ECM) is observed.
Keywords: articular chondrocyte; cell cycle; cyclin-dependent kinases; osteoarthritis; pentosan polysulfate.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures

Similar articles
-
Pentosan polysulfate sodium promotes redifferentiation to the original phenotype in micromass-cultured canine articular chondrocytes and exerts molecular weight-dependent effects.J Vet Med Sci. 2023 Jun 14;85(6):680-690. doi: 10.1292/jvms.22-0567. Epub 2023 May 8. J Vet Med Sci. 2023. PMID: 37150611 Free PMC article.
-
Effects of pentosan polysulfate and polysulfated glycosaminoglycan on chondrogenesis of canine bone marrow-derived mesenchymal stem cells in alginate and micromass culture.J Vet Med Sci. 2017 Jul 7;79(7):1182-1190. doi: 10.1292/jvms.17-0084. Epub 2017 May 27. J Vet Med Sci. 2017. PMID: 28552861 Free PMC article.
-
Pentosan polysulfate inhibits IL-1β-induced iNOS, c-Jun and HIF-1α upregulation in canine articular chondrocytes.PLoS One. 2017 May 4;12(5):e0177144. doi: 10.1371/journal.pone.0177144. eCollection 2017. PLoS One. 2017. PMID: 28472120 Free PMC article.
-
The pathobiology of osteoarthritis and the rationale for the use of pentosan polysulfate for its treatment.Semin Arthritis Rheum. 1999 Feb;28(4):211-67. doi: 10.1016/s0049-0172(99)80021-3. Semin Arthritis Rheum. 1999. PMID: 10073500 Review.
-
Pentosan Polysulfate, a Semisynthetic Heparinoid Disease-Modifying Osteoarthritic Drug with Roles in Intervertebral Disc Repair Biology Emulating the Stem Cell Instructive and Tissue Reparative Properties of Heparan Sulfate.Stem Cells Dev. 2022 Aug;31(15-16):406-430. doi: 10.1089/scd.2022.0007. Epub 2022 Mar 14. Stem Cells Dev. 2022. PMID: 35102748 Review.
Cited by
-
Pentosan polysulfate sodium promotes redifferentiation to the original phenotype in micromass-cultured canine articular chondrocytes and exerts molecular weight-dependent effects.J Vet Med Sci. 2023 Jun 14;85(6):680-690. doi: 10.1292/jvms.22-0567. Epub 2023 May 8. J Vet Med Sci. 2023. PMID: 37150611 Free PMC article.
-
HIF-1α in Osteoarthritis: From Pathogenesis to Therapeutic Implications.Front Pharmacol. 2022 Jul 5;13:927126. doi: 10.3389/fphar.2022.927126. eCollection 2022. Front Pharmacol. 2022. PMID: 35865944 Free PMC article. Review.
References
-
- Berridge M. J.2014. Cell cycle and proliferation. Cell Signal. Biol. 6: csb0001009. doi: 10.1042/csb0001009 - DOI

