LRRK2 mediates axon development by regulating Frizzled3 phosphorylation and growth cone-growth cone communication
- PMID: 32641508
- PMCID: PMC7395514
- DOI: 10.1073/pnas.1921878117
LRRK2 mediates axon development by regulating Frizzled3 phosphorylation and growth cone-growth cone communication
Abstract
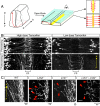
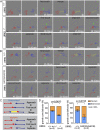
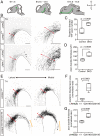
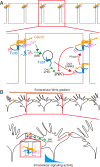
Axon-axon interactions are essential for axon guidance during nervous system wiring. However, it is unknown whether and how the growth cones communicate with each other while sensing and responding to guidance cues. We found that the Parkinson's disease gene, leucine-rich repeat kinase 2 (LRRK2), has an unexpected role in growth cone-growth cone communication. The LRRK2 protein acts as a scaffold and induces Frizzled3 hyperphosphorylation indirectly by recruiting other kinases and also directly phosphorylates Frizzled3 on threonine 598 (T598). In LRRK1 or LRRK2 single knockout, LRRK1/2 double knockout, and LRRK2 G2019S knockin, the postcrossing spinal cord commissural axons are disorganized and showed anterior-posterior guidance errors after midline crossing. Growth cones from either LRRK2 knockout or G2019S knockin mice showed altered interactions, suggesting impaired communication. Intercellular interaction between Frizzled3 and Vangl2 is essential for planar cell polarity signaling. We show here that this interaction is regulated by phosphorylation of Frizzled3 at T598 and can be regulated by LRRK2 in a kinase activity-dependent way. In the LRRK1/2 double knockout or LRRK2 G2019S knockin, the dopaminergic axon bundle in the midbrain was significantly widened and appeared disorganized, showing aberrant posterior-directed growth. Our findings demonstrate that LRRK2 regulates growth cone-growth cone communication in axon guidance and that both loss-of-function mutation and a gain-of-function mutation (G2019S) cause axon guidance defects in development.
Keywords: Frizzled3–Vangl2 interaction; LRRK2; Wnt/planar cell polarity; axon guidance; growth cone–growth cone interaction.
Copyright © 2020 the Author(s). Published by PNAS.
Conflict of interest statement
Competing interest statement: Y.Z. is the founder of VersaPeutics and has equity, compensation, and interim managerial role. The terms of this arrangement have been reviewed and approved by the University of California San Diego in accordance with its conflict of interest policies.
Figures




Similar articles
-
Inter-growth cone communication mediated by planar cell polarity pathway in axon guidance.Dev Biol. 2022 Oct;490:50-52. doi: 10.1016/j.ydbio.2022.06.016. Epub 2022 Jul 1. Dev Biol. 2022. PMID: 35788000
-
Antagonistic functions of Dishevelleds regulate Frizzled3 endocytosis via filopodia tips in Wnt-mediated growth cone guidance.J Neurosci. 2013 Dec 4;33(49):19071-85. doi: 10.1523/JNEUROSCI.2800-13.2013. J Neurosci. 2013. PMID: 24305805 Free PMC article.
-
Vangl2 promotes Wnt/planar cell polarity-like signaling by antagonizing Dvl1-mediated feedback inhibition in growth cone guidance.Dev Cell. 2011 Feb 15;20(2):177-91. doi: 10.1016/j.devcel.2011.01.002. Dev Cell. 2011. PMID: 21316586 Free PMC article.
-
Functional and behavioral consequences of Parkinson's disease-associated LRRK2-G2019S mutation.Biochem Soc Trans. 2018 Dec 17;46(6):1697-1705. doi: 10.1042/BST20180468. Epub 2018 Dec 4. Biochem Soc Trans. 2018. PMID: 30514770 Free PMC article. Review.
-
LRRK2 Phosphorylation: Behind the Scenes.Neuroscientist. 2018 Oct;24(5):486-500. doi: 10.1177/1073858418756309. Epub 2018 Jan 31. Neuroscientist. 2018. PMID: 29385885 Review.
Cited by
-
Structural insights into Frizzled3 through nanobody modulators.Nat Commun. 2024 Aug 22;15(1):7228. doi: 10.1038/s41467-024-51451-1. Nat Commun. 2024. PMID: 39174501 Free PMC article.
-
UNC-16 interacts with LRK-1 and WDFY-3 to regulate the termination of axon growth.bioRxiv [Preprint]. 2024 Feb 16:2024.02.15.580526. doi: 10.1101/2024.02.15.580526. bioRxiv. 2024. Update in: Genetics. 2024 Jun 5;227(2):iyae053. doi: 10.1093/genetics/iyae053 PMID: 38405875 Free PMC article. Updated. Preprint.
-
A Role for Frizzled and Their Post-Translational Modifications in the Mammalian Central Nervous System.Front Cell Dev Biol. 2021 Aug 3;9:692888. doi: 10.3389/fcell.2021.692888. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34414184 Free PMC article. Review.
-
Prickle promotes the formation and maintenance of glutamatergic synapses by stabilizing the intercellular planar cell polarity complex.Sci Adv. 2021 Oct 8;7(41):eabh2974. doi: 10.1126/sciadv.abh2974. Epub 2021 Oct 6. Sci Adv. 2021. PMID: 34613779 Free PMC article.
-
Neuronal guidance genes in health and diseases.Protein Cell. 2023 Apr 21;14(4):238-261. doi: 10.1093/procel/pwac030. Protein Cell. 2023. PMID: 36942388 Free PMC article. Review.
References
-
- Stoeckli E. T., Understanding axon guidance: Are we nearly there yet? Development 145, dev151415 (2018). - PubMed
-
- Wang L., Marquardt T., What axons tell each other: Axon-axon signaling in nerve and circuit assembly. Curr. Opin. Neurobiol. 23, 974–982 (2013). - PubMed
-
- Lyuksyutova A. I. et al. ., Anterior-posterior guidance of commissural axons by Wnt-frizzled signaling. Science 302, 1984–1988 (2003). - PubMed
-
- Yoshikawa S., McKinnon R. D., Kokel M., Thomas J. B., Wnt-mediated axon guidance via the Drosophila Derailed receptor. Nature 422, 583–588 (2003). - PubMed
-
- Liu Y. et al. ., Ryk-mediated Wnt repulsion regulates posterior-directed growth of corticospinal tract. Nat. Neurosci. 8, 1151–1159 (2005). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

