Transposable elements in Drosophila
- PMID: 32636946
- PMCID: PMC7334843
- DOI: 10.1186/s13100-020-00213-z
Transposable elements in Drosophila
Abstract
Drosophila has been studied as a biological model for many years and many discoveries in biology rely on this species. Research on transposable elements (TEs) is not an exception. Drosophila has contributed significantly to our knowledge on the mechanisms of transposition and their regulation, but above all, it was one of the first organisms on which genetic and genomic studies of populations were done. In this review article, in a very broad way, we will approach the TEs of Drosophila with a historical hindsight as well as recent discoveries in the field.
Keywords: Drosophila; Population genomics; epigenetics; intra and interspecific TE diversity.
© The Author(s) 2020.
Conflict of interest statement
Competing interestsThe authors declare that they have no competing interests
Figures


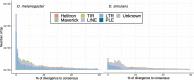
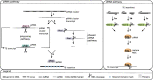
Similar articles
-
Interspecific hybridization as a genomic stressor inducing mobilization of transposable elements in Drosophila.Mob Genet Elements. 2014 Aug 8;4:e34394. doi: 10.4161/mge.34394. eCollection 2014. Mob Genet Elements. 2014. PMID: 25136509 Free PMC article.
-
Species-specific chromatin landscape determines how transposable elements shape genome evolution.Elife. 2022 Aug 23;11:e81567. doi: 10.7554/eLife.81567. Elife. 2022. PMID: 35997258 Free PMC article.
-
Transposable elements in natural populations of Drosophila melanogaster.Philos Trans R Soc Lond B Biol Sci. 2010 Apr 27;365(1544):1219-28. doi: 10.1098/rstb.2009.0318. Philos Trans R Soc Lond B Biol Sci. 2010. PMID: 20308097 Free PMC article. Review.
-
Silencing of Transposable Elements by piRNAs in Drosophila: An Evolutionary Perspective.Genomics Proteomics Bioinformatics. 2017 Jun;15(3):164-176. doi: 10.1016/j.gpb.2017.01.006. Epub 2017 Jun 8. Genomics Proteomics Bioinformatics. 2017. PMID: 28602845 Free PMC article. Review.
-
The Transposable Elements of the Drosophila serrata Reference Panel.Genome Biol Evol. 2021 Sep 1;13(9):evab100. doi: 10.1093/gbe/evab100. Genome Biol Evol. 2021. PMID: 33950180 Free PMC article.
Cited by
-
The impact of insertion bias into piRNA clusters on the invasion of transposable elements.bioRxiv [Preprint]. 2024 Oct 18:2024.10.06.616898. doi: 10.1101/2024.10.06.616898. bioRxiv. 2024. PMID: 39464153 Free PMC article. Preprint.
-
Making the Genome Huge: The Case of Triatoma delpontei, a Triatominae Species with More than 50% of Its Genome Full of Satellite DNA.Genes (Basel). 2023 Jan 31;14(2):371. doi: 10.3390/genes14020371. Genes (Basel). 2023. PMID: 36833298 Free PMC article.
-
The Worldwide Invasion of Drosophila suzukii Is Accompanied by a Large Increase of Transposable Element Load and a Small Number of Putatively Adaptive Insertions.Mol Biol Evol. 2021 Sep 27;38(10):4252-4267. doi: 10.1093/molbev/msab155. Mol Biol Evol. 2021. PMID: 34021759 Free PMC article.
-
Chemotherapy Drugs Act Differently in the Expression and Somatic Mobilization of the mariner Transposable Element in Drosophila simulans.Genes (Basel). 2022 Dec 16;13(12):2374. doi: 10.3390/genes13122374. Genes (Basel). 2022. PMID: 36553641 Free PMC article.
-
Variation in mutation, recombination, and transposition rates in Drosophila melanogaster and Drosophila simulans.Genome Res. 2023 Apr;33(4):587-598. doi: 10.1101/gr.277383.122. Epub 2023 Apr 10. Genome Res. 2023. PMID: 37037625 Free PMC article.
References
-
- Doolittle WF, Sapienza C. Selfish genes, the phenotype paradigm and genome evolution. Nature. 1980;284:601–603. - PubMed
-
- Orgel LE, Crick FHC. Selfish DNA: the ultimate parasite. Nature. 1980;284:604–607. - PubMed
-
- Charlesworth B, Charlesworth D. The population dynamics of transposable elements. Genet Res. 1983;42:1–27.
-
- Feschotte C, Jiang N, Wessler SR. Plant transposable elements: where genetics meets genomics. Nat Rev Genet. 2002;3:329–341. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

