The Tubulin Code in Microtubule Dynamics and Information Encoding
- PMID: 32634400
- PMCID: PMC11042690
- DOI: 10.1016/j.devcel.2020.06.008
The Tubulin Code in Microtubule Dynamics and Information Encoding
Abstract
Microtubules are non-covalent mesoscale polymers central to the eukaryotic cytoskeleton. Microtubule structure, dynamics, and mechanics are modulated by a cell's choice of tubulin isoforms and post-translational modifications, a "tubulin code," which is thought to support the diverse morphology and dynamics of microtubule arrays across various cell types, cell cycle, and developmental stages. We give a brief historical overview of research into tubulin diversity and highlight recent progress toward uncovering the mechanistic underpinnings of the tubulin code. As a large number of essential pathways converge upon the microtubule cytoskeleton, understanding how cells utilize tubulin diversity is crucial to understanding cellular physiology and disease.
Keywords: CCP; TTLL; detyrosination; dynein; glutamylation; glycylation; kinesin; microtubule; microtubule associated proteins; motors; severing; tubulin code; tubulin isoforms; tubulin post-translational modifications; tubulin tyrosine ligase; tyrosination.
Published by Elsevier Inc.
Figures

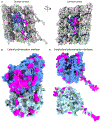
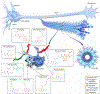

Similar articles
-
The Tubulin Code, from Molecules to Health and Disease.Annu Rev Cell Dev Biol. 2023 Oct 16;39:331-361. doi: 10.1146/annurev-cellbio-030123-032748. Annu Rev Cell Dev Biol. 2023. PMID: 37843925 Review.
-
Combinatorial and antagonistic effects of tubulin glutamylation and glycylation on katanin microtubule severing.Dev Cell. 2022 Nov 7;57(21):2497-2513.e6. doi: 10.1016/j.devcel.2022.10.003. Dev Cell. 2022. PMID: 36347241 Free PMC article.
-
The tubulin code and its role in controlling microtubule properties and functions.Nat Rev Mol Cell Biol. 2020 Jun;21(6):307-326. doi: 10.1038/s41580-020-0214-3. Epub 2020 Feb 27. Nat Rev Mol Cell Biol. 2020. PMID: 32107477 Review.
-
The tubulin code at a glance.J Cell Sci. 2017 Apr 15;130(8):1347-1353. doi: 10.1242/jcs.199471. Epub 2017 Mar 21. J Cell Sci. 2017. PMID: 28325758 Review.
-
Writing and Reading the Tubulin Code.J Biol Chem. 2015 Jul 10;290(28):17163-72. doi: 10.1074/jbc.R115.637447. Epub 2015 May 8. J Biol Chem. 2015. PMID: 25957412 Free PMC article. Review.
Cited by
-
Cracking the tubulin code: enzyme structures offer clues to microtubule control.Nature. 2024 Sep 11. doi: 10.1038/d41586-024-02822-7. Online ahead of print. Nature. 2024. PMID: 39261685 No abstract available.
-
The synaptic life of microtubules.Curr Opin Neurobiol. 2021 Aug;69:113-123. doi: 10.1016/j.conb.2021.03.004. Epub 2021 Apr 16. Curr Opin Neurobiol. 2021. PMID: 33873059 Free PMC article. Review.
-
Telomere shortening impairs alveolar regeneration.Cell Prolif. 2022 Apr;55(4):e13211. doi: 10.1111/cpr.13211. Epub 2022 Mar 11. Cell Prolif. 2022. PMID: 35274784 Free PMC article.
-
Manipulation of Host Microtubule Networks by Viral Microtubule-Associated Proteins.Viruses. 2022 May 6;14(5):979. doi: 10.3390/v14050979. Viruses. 2022. PMID: 35632720 Free PMC article. Review.
-
Cytoskeleton and Associated Proteins: Pleiotropic JNK Substrates and Regulators.Int J Mol Sci. 2021 Aug 4;22(16):8375. doi: 10.3390/ijms22168375. Int J Mol Sci. 2021. PMID: 34445080 Free PMC article. Review.
References
-
- Aillaud C., Bosc C., Peris L., Bosson A., Heemeryck P., Van Dij J., Le Friec J., Boulan B., Vossier F., and Sanman LE. (2017). Vasohibins/SVBP are tubulin carboxypeptidases (TCPs) that regulate neuron differentiation. Science 358, 1448–1453. - PubMed
-
- Arce CA, Rodriguez JA, Barra HS, and Caputo R (1975). Incorporation of L-tyrosine, L-phenylalanine and L-3,4-dihydroxyphenylalanine as single units into rat brain tubulin. Eur J Biochem 59, 145–149. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

