Cyclophilin D: An Integrator of Mitochondrial Function
- PMID: 32625108
- PMCID: PMC7311779
- DOI: 10.3389/fphys.2020.00595
Cyclophilin D: An Integrator of Mitochondrial Function
Abstract
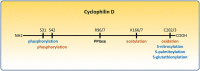
Cyclophilin D (CypD) is a mitochondrial peptidyl-prolyl cis-trans isomerase, well-known for regulating the mitochondrial permeability transition pore (PTP), a nonspecific large conductance pore whose opening leads to cell death and has been implicated in ischemia/reperfusion injury in multiple organs, in neurodegenerative disorders, and in muscular dystrophies. While the main target of CypD is a matter of ongoing research, inhibiting CypD protects in models of those diseases making it an interesting therapeutic target. The present review focuses on post-translational modifications of CypD that have been identified by recent studies, which can alter the regulation of the PTP and contribute to understanding the mechanisms of action of CypD.
Keywords: ATP synthase; cyclophilin D; cyclosporine A; mitochondrial function; peptidyl-prolyl cis-trans isomerase; permeability transition pore.
Copyright © 2020 Amanakis and Murphy.
Figures
Similar articles
-
Cyclophilin D-mediated Mitochondrial Permeability Transition Regulates Mitochondrial Function.Curr Pharm Des. 2023;29(8):620-629. doi: 10.2174/1381612829666230313111314. Curr Pharm Des. 2023. PMID: 36915987 Review.
-
Cysteine 202 of cyclophilin D is a site of multiple post-translational modifications and plays a role in cardioprotection.Cardiovasc Res. 2021 Jan 1;117(1):212-223. doi: 10.1093/cvr/cvaa053. Cardiovasc Res. 2021. PMID: 32129829 Free PMC article.
-
Small-Molecule Inhibitors of Cyclophilins Block Opening of the Mitochondrial Permeability Transition Pore and Protect Mice From Hepatic Ischemia/Reperfusion Injury.Gastroenterology. 2019 Nov;157(5):1368-1382. doi: 10.1053/j.gastro.2019.07.026. Epub 2019 Jul 20. Gastroenterology. 2019. PMID: 31336123
-
Mitochondrial ATP synthase inhibitory factor 1 interacts with the p53-cyclophilin D complex and promotes opening of the permeability transition pore.J Biol Chem. 2022 May;298(5):101858. doi: 10.1016/j.jbc.2022.101858. Epub 2022 Mar 23. J Biol Chem. 2022. PMID: 35337801 Free PMC article.
-
Cyclophilin D in Mitochondrial Dysfunction: A Key Player in Neurodegeneration?Biomolecules. 2023 Aug 18;13(8):1265. doi: 10.3390/biom13081265. Biomolecules. 2023. PMID: 37627330 Free PMC article. Review.
Cited by
-
The correlation between mitochondria-associated endoplasmic reticulum membranes (MAMs) and Ca2+ transport in the pathogenesis of diseases.Acta Pharmacol Sin. 2025 Feb;46(2):271-291. doi: 10.1038/s41401-024-01359-9. Epub 2024 Aug 8. Acta Pharmacol Sin. 2025. PMID: 39117969 Review.
-
Activated CYPD in COPD: Filling in the Puzzle of how Perturbed Epithelial Respiration Leads to Disturbed Respiratory Function.Lung. 2023 Jun;201(3):251-252. doi: 10.1007/s00408-023-00623-9. Epub 2023 Jun 1. Lung. 2023. PMID: 37261530 No abstract available.
-
Remodeling of Liver and Plasma Lipidomes in Mice Lacking Cyclophilin D.Int J Mol Sci. 2022 Sep 24;23(19):11274. doi: 10.3390/ijms231911274. Int J Mol Sci. 2022. PMID: 36232575 Free PMC article.
-
(-)-Epigallocatechin-3-gallate Directly Binds Cyclophilin D: A Potential Mechanism for Mitochondrial Protection.Molecules. 2022 Dec 7;27(24):8661. doi: 10.3390/molecules27248661. Molecules. 2022. PMID: 36557795 Free PMC article.
-
Association of cyclophilins and cardiovascular risk factors in coronary artery disease.Front Physiol. 2023 Mar 1;14:1127468. doi: 10.3389/fphys.2023.1127468. eCollection 2023. Front Physiol. 2023. PMID: 36935755 Free PMC article.
References
-
- Agarwal A., Wu P. H., Hughes E. G., Fukaya M., Tischfield M. A., Langseth A. J., et al. . (2017). Transient opening of the mitochondrial permeability transition pore induces microdomain calcium transients in astrocyte processes. Neuron 93, 587–605. 10.1016/j.neuron.2016.12.034, PMID: - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

