Potential Mechanism of Dermal Wound Treatment With Preparations From the Skin Gel of Arabian Gulf Catfish: A Unique Furan Fatty Acid (F6) and Cholesta-3,5-Diene (S5) Recruit Neutrophils and Fibroblasts to Promote Wound Healing
- PMID: 32625093
- PMCID: PMC7314935
- DOI: 10.3389/fphar.2020.00899
Potential Mechanism of Dermal Wound Treatment With Preparations From the Skin Gel of Arabian Gulf Catfish: A Unique Furan Fatty Acid (F6) and Cholesta-3,5-Diene (S5) Recruit Neutrophils and Fibroblasts to Promote Wound Healing
Abstract
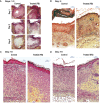
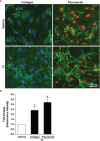
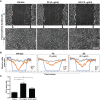
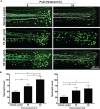
Preparations from Arabian Gulf catfish (Arius bilineatus, Val) epidermal gel secretion (PCEGS) effectively heal chronic wounds in diabetic patients. However, specific lipid components of PCEGS that are responsible for various aspects of wound healing are unknown. Here, we report for the first time that, i) a unique preparation containing only proteins and lipids (Fraction B, FB), derived from the PCEGS accelerated the healing of experimental dermal wounds in female rats (transdermal punch biopsy) in vivo. Histological analyses showed that topical treatment of these wounds with FB promoted the migration of fibroblasts, facilitated the production of extracellular matrix (collagen, fibronectin), induced capillary formation and recruitment of immune cells, and accelerated overall wound healing by day 4 (tested at 1, 2, 3, 4, and 10 days; n=15 for vehicle; n=15 for FB treatment), ii) the lipids responsible for different stages of wound healing were separated into a protein-free bioactive lipid fraction, Ft, which contained a few common long-chain fatty acids, a unique furan fatty acid (F6) and a cholesterol metabolite, cholesta-3,5-diene (S5). Ft (the partially purified lipid fraction of PCEGS), and F6 and S5 present in Ft, proved to be bioactive for wound healing in human dermal fibroblasts. Ft increased the production and extracellular deposition of collagen and fibronectin, ex vivo, iii) Ft and its subcomponents, pure F6 and S5, also promoted human dermal fibroblast migration into the scratch wound gaps, ex vivo, iv) Ft, F6, and S5 promoted the recruitment of neutrophils (Green fluorescence protein labeled) to the site of injury in the transected tailfins of transgenic zebrafish, in vivo, v) Ft, but not F6 or S5, promoted the regeneration of tissues at the wound site in the transgenic zebrafish tailfin, in vivo. Therefore, we conclude that lipid fraction Ft from PCEGS contains the components necessary to promote complete wound healing, and F6 and S5 are responsible for promoting fibroblast and neutrophil recruitment to the site of wounds.
Keywords: Fraction-B; Gulf catfish lipids; cholesta-3,5-diene; fibroblasts; furan F-acid; histology; leukocyte; wound healing.
Copyright © 2020 Al-Hassan, Hinek, Renno, Wang, Liu, Guan, Wen, Litvack, Lindenmaier, Afzal, Paul, Oommen, Nair, Kumar, Khan, Palaniyar and Pace-Asciak.
Figures


Similar articles
-
Catfish Epidermal Preparation Accelerates Healing of Damaged Nerve in a Sciatic Nerve Crush Injury Rat Model.Front Pharmacol. 2021 Apr 14;12:632028. doi: 10.3389/fphar.2021.632028. eCollection 2021. Front Pharmacol. 2021. PMID: 33986668 Free PMC article.
-
Oxidized Cholesterol Derivatives in Fraction B Prepared from Gulf Catfish (Arius bilineatus, Val.) Skin Regulate Calcium Response and Neutrophil Extracellular Traps (NETs) Formation.Biomedicines. 2024 Jun 21;12(7):1380. doi: 10.3390/biomedicines12071380. Biomedicines. 2024. PMID: 39061953 Free PMC article.
-
Furanoid F-Acid F6 Uniquely Induces NETosis Compared to C16 and C18 Fatty Acids in Human Neutrophils.Biomolecules. 2018 Nov 13;8(4):144. doi: 10.3390/biom8040144. Biomolecules. 2018. PMID: 30428625 Free PMC article.
-
[The modern approach to wound treatment].Med Pregl. 2000 Jul-Aug;53(7-8):363-8. Med Pregl. 2000. PMID: 11214479 Review. Croatian.
-
Extracellular Matrix and Dermal Fibroblast Function in the Healing Wound.Adv Wound Care (New Rochelle). 2016 Mar 1;5(3):119-136. doi: 10.1089/wound.2014.0561. Adv Wound Care (New Rochelle). 2016. PMID: 26989578 Free PMC article. Review.
Cited by
-
Gentiopicroside injection promotes the healing of pressure injury wounds by upregulating the expression of bFGFR1.Rev Esc Enferm USP. 2024 Jul 8;58:e20230183. doi: 10.1590/1980-220X-REEUSP-2023-0183en. eCollection 2024. Rev Esc Enferm USP. 2024. PMID: 38985820 Free PMC article.
-
Antioxidant and Anti-Inflammatory Properties of Quail Yolk Oil via Upregulation of Superoxide Dismutase 1 and Catalase Genes and Downregulation of EIGER and Unpaired 2 Genes in a D. melanogaster Model.Antioxidants (Basel). 2024 Jan 5;13(1):75. doi: 10.3390/antiox13010075. Antioxidants (Basel). 2024. PMID: 38247499 Free PMC article.
-
Moschus ameliorates glutamate-induced cellular damage by regulating autophagy and apoptosis pathway.Sci Rep. 2023 Oct 30;13(1):18586. doi: 10.1038/s41598-023-45878-7. Sci Rep. 2023. PMID: 37903904 Free PMC article.
-
Catfish Epidermal Preparation Accelerates Healing of Damaged Nerve in a Sciatic Nerve Crush Injury Rat Model.Front Pharmacol. 2021 Apr 14;12:632028. doi: 10.3389/fphar.2021.632028. eCollection 2021. Front Pharmacol. 2021. PMID: 33986668 Free PMC article.
-
Neuroregeneration of injured peripheral nerve by fraction B of catfish epidermal secretions through the reversal of the apoptotic pathway and DNA damage.Front Pharmacol. 2023 Jan 16;14:1085314. doi: 10.3389/fphar.2023.1085314. eCollection 2023. Front Pharmacol. 2023. PMID: 36726586 Free PMC article.
References
-
- Al-Hassan J. M., Thomson M., Criddle R. S. (1983). Accelerated wound healing by a preparation from skin of the Arabian Gulf catfish. Lancet 1 (8332), 1043–1044. - PubMed
-
- Al-Hassan J. M., Dyson M., Young S. R., Thomson M., Criddle R. S. (1991). Acceleration of wound healing responses induced by preparations from the epidermal secretions of the arabian gulf catfish (Arius Bilineatus, Valenciennes). J. Wilderness Med. 2, 153–163. 10.1580/0953-9859-2.3.153 - DOI
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

