Inhibition of miR-25 attenuates doxorubicin-induced apoptosis, reactive oxygen species production and DNA damage by targeting PTEN
- PMID: 32624698
- PMCID: PMC7330660
- DOI: 10.7150/ijms.41980
Inhibition of miR-25 attenuates doxorubicin-induced apoptosis, reactive oxygen species production and DNA damage by targeting PTEN
Abstract
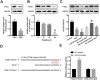
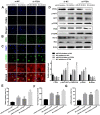
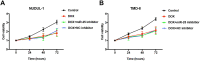
Background: Doxorubicin (DOX) is one of the widely used anti-cancer drugs, whereas it can induce irreversible cardiac injury in a dose-dependent manner which limits its utility in clinic. Our study aimed to investigate the relationship between miR-25 and DOX-induced cardiac injury and its underlying mechanism. Methods: Mice and H9c2 cells were exposed to DOX. The overexpressed or knockdown of miR-25 in H9c2 cells was achieved by miR-25 mimic or inhibitor and the efficiency of transfection was identified by qRT-PCR or Western blotting. Cell viability, apoptotic cell rate, and levels of apoptosis-related proteins were determined by CCK-8, flow cytometry, and Western blotting, respectively. Furthermore, Western blotting and immunofluorescence staining (IF) were performed to assess the expression levels of reactive oxygen species and degree of DNA damage. Results: As a result, DOX significantly upregulated miR-25 expression in mice and H9c2 cells and reduced cell viability and increased cell apoptosis in vitro and in vivo. miR-25 overexpression expedited cell injury induced by DOX in H9c2 cells demonstrated by the increased cell apoptosis and reactive oxygen species (ROS) production, whereas miR-25 inhibition attenuated the cell injury. Furthermore, miR-25 negatively controlled the expression of phosphatase and tensin homolog deleted on chromosome 10 (PTEN). Intervention the expression of PTEN using si-PTEN reversed the beneficial effects of miR-25 inhibition on DOX-injured H9c2 cells. Conclusion: In conclusion, this study demonstrated that miR-25 is involved in DOX-induced cell damage through the regulation of PTEN expression.
Keywords: H9c2 cells; PTEN; doxorubicin-induced cardiotoxicity; miR-25.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
Similar articles
-
Inhibition of miR-23a attenuates doxorubicin-induced mitochondria-dependent cardiomyocyte apoptosis by targeting the PGC-1α/Drp1 pathway.Toxicol Appl Pharmacol. 2019 Apr 15;369:73-81. doi: 10.1016/j.taap.2019.02.016. Epub 2019 Mar 1. Toxicol Appl Pharmacol. 2019. PMID: 30831132
-
MiR-15b-5p is Involved in Doxorubicin-Induced Cardiotoxicity via Inhibiting Bmpr1a Signal in H9c2 Cardiomyocyte.Cardiovasc Toxicol. 2019 Jun;19(3):264-275. doi: 10.1007/s12012-018-9495-6. Cardiovasc Toxicol. 2019. PMID: 30535663
-
MiR-24-3p modulates cardiac function in doxorubicin -induced heart failure via the Sp1/PI3K signaling pathway.Cell Signal. 2024 Dec;124:111407. doi: 10.1016/j.cellsig.2024.111407. Epub 2024 Sep 14. Cell Signal. 2024. PMID: 39278455
-
Glycyrrhiza glabra (Licorice) root extract attenuates doxorubicin-induced cardiotoxicity via alleviating oxidative stress and stabilising the cardiac health in H9c2 cardiomyocytes.J Ethnopharmacol. 2020 Aug 10;258:112690. doi: 10.1016/j.jep.2020.112690. Epub 2020 Feb 24. J Ethnopharmacol. 2020. PMID: 32105749
-
Doxorubicin-An Agent with Multiple Mechanisms of Anticancer Activity.Cells. 2023 Feb 19;12(4):659. doi: 10.3390/cells12040659. Cells. 2023. PMID: 36831326 Free PMC article. Review.
Cited by
-
Rhabdomyosarcoma: Current Therapy, Challenges, and Future Approaches to Treatment Strategies.Cancers (Basel). 2023 Nov 2;15(21):5269. doi: 10.3390/cancers15215269. Cancers (Basel). 2023. PMID: 37958442 Free PMC article. Review.
-
Epigenetic Mechanisms Involved in the Cardiovascular Toxicity of Anticancer Drugs.Front Cardiovasc Med. 2021 Apr 27;8:658900. doi: 10.3389/fcvm.2021.658900. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 33987212 Free PMC article. Review.
-
Silencing of microRNA-106b-5p prevents doxorubicin-mediated cardiotoxicity through modulation of the PR55α/YY1/sST2 signaling axis.Mol Ther Nucleic Acids. 2023 May 3;32:704-720. doi: 10.1016/j.omtn.2023.04.031. eCollection 2023 Jun 13. Mol Ther Nucleic Acids. 2023. PMID: 37234747 Free PMC article.
-
MicroRNAs in doxorubicin-induced cardiotoxicity: The DNA damage response.Front Pharmacol. 2022 Nov 21;13:1055911. doi: 10.3389/fphar.2022.1055911. eCollection 2022. Front Pharmacol. 2022. PMID: 36479202 Free PMC article. Review.
-
The anti-tumor and renoprotection study of E-[c(RGDfK)2]/folic acid co-modified nanostructured lipid carrier loaded with doxorubicin hydrochloride/salvianolic acid A.J Nanobiotechnology. 2022 Sep 24;20(1):425. doi: 10.1186/s12951-022-01628-x. J Nanobiotechnology. 2022. PMID: 36153589 Free PMC article.
References
-
- Cardinale D, Colombo A, Bacchiani G. et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;131(22):1981–1988. - PubMed
-
- M L, JAM K, A vR. et al. Cardiovascular adverse events in patients with non-Hodgkin lymphoma treated with first-line cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or CHOP with rituximab (R-CHOP): a systematic review and meta-analysis. The Lancet Haematology. 2020;7(4):e295–e308. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

