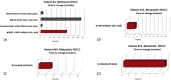
Molecular matching and treatment strategies for advanced stage lung cancer at Dartmouth-Hitchcock Medical Center: A three-year review of a Molecular Tumor Board
- PMID: 32613070
- PMCID: PMC7322356
- DOI: 10.1016/j.plabm.2020.e00174
Molecular matching and treatment strategies for advanced stage lung cancer at Dartmouth-Hitchcock Medical Center: A three-year review of a Molecular Tumor Board
Abstract
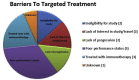
Matching of actionable tumor mutations with targeted therapy increases response rates and prolongs survival in lung cancer patients. Drug development and trials targeting genetic alterations are expanding rapidly. We describe the role of a Molecular Tumor Board (MTB) in the design of molecularly informed treatment strategies in our lung cancer patient population. Tumor DNA was sequenced using a 50-gene targeted next-generation sequencing panel. Cases were evaluated by a multidisciplinary MTB who suggested a course of treatment based on each patient's molecular findings. During a three-year period, 21 lung cancer patients were presented at the MTB. All patients lacked common activating EGFR mutations and ALK rearrangements. One patient had Stage IIIb disease; all others were Stage IV; 18 patients had received ≥1 prior line of therapy (range 0-5). Suggestions for treatment with a targeted therapy were made for 19/21 (90.5%) patients, and four patients (21%) underwent treatment with a targeted agent, two as part of a clinical trial. Identified barriers to treatment with targeted therapy included: ineligibility for clinical trials (n = 2), lack of interest in study/distance to travel (n = 2), lack of disease progression (n = 2), poor performance status (n = 5), decision to treat next with immunotherapy (n = 3), and unknown (n = 1). For the majority of lung cancer patients, the MTB provided recommendations based on tumor genetic profiles. Identified barriers to treatment suggest that presentation to the MTB at earlier stages of disease may increase the number of patients eligible for treatment with a genetically informed targeted agent.
Keywords: Lung cancer; Molecular tumor board; Personalized medicine; Targeted therapies; Tumor genotyping.
© 2020 Published by Elsevier B.V.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures
Similar articles
-
Implementation of a Molecular Tumor Board: The Impact on Treatment Decisions for 35 Patients Evaluated at Dartmouth-Hitchcock Medical Center.Oncologist. 2015 Sep;20(9):1011-8. doi: 10.1634/theoncologist.2015-0097. Epub 2015 Jul 23. Oncologist. 2015. PMID: 26205736 Free PMC article.
-
Implementation of Precision Oncology for Patients with Metastatic Breast Cancer in an Interdisciplinary MTB Setting.Diagnostics (Basel). 2021 Apr 20;11(4):733. doi: 10.3390/diagnostics11040733. Diagnostics (Basel). 2021. PMID: 33924134 Free PMC article.
-
Establishment of a Molecular Tumor Board (MTB) and Uptake of Recommendations in a Community Setting.J Pers Med. 2020 Nov 27;10(4):252. doi: 10.3390/jpm10040252. J Pers Med. 2020. PMID: 33260805 Free PMC article.
-
Implementation and utilization of the molecular tumor board to guide precision medicine.Oncotarget. 2017 Jun 14;8(34):57845-57854. doi: 10.18632/oncotarget.18471. eCollection 2017 Aug 22. Oncotarget. 2017. PMID: 28915716 Free PMC article. Review.
-
Overview of resistance to systemic therapy in patients with breast cancer.Adv Exp Med Biol. 2007;608:1-22. doi: 10.1007/978-0-387-74039-3_1. Adv Exp Med Biol. 2007. PMID: 17993229 Review.
Cited by
-
Lessons learned: the first consecutive 1000 patients of the CCCMunichLMU Molecular Tumor Board.J Cancer Res Clin Oncol. 2023 May;149(5):1905-1915. doi: 10.1007/s00432-022-04165-0. Epub 2022 Jul 7. J Cancer Res Clin Oncol. 2023. PMID: 35796778 Free PMC article. Review.
-
Validation and Implementation of a Somatic-Only Tumor Exome for Routine Clinical Application.J Mol Diagn. 2024 Sep;26(9):815-824. doi: 10.1016/j.jmoldx.2024.05.013. Epub 2024 Jul 6. J Mol Diagn. 2024. PMID: 38972591
References
-
- Howlader N., Noone A.M., Krapcho M., Miller D., Bishop K., Altekruse S.F., Kosary C.L., Yu M., Ruhl J., Tatalovich Z., Mariotto A., Lewis D.R., Chen H.S., Feuer E.J., Cronin K.A., editors. SEER Cancer Statistics Review. National Cancer Institute; Bethesda, MD: 1975-2013. http://seer.cancer.gov/csr/1975_2013/ based on November 2015 SEER data submission, posted to the SEER web site, April 2016.
-
- Lindeman N.I., Cagle P.T., Beasley M.B., Chitale D.A., Dacic S., Giaccone G., Jenkins R.B., Kwiatkowski D.J., Saldivar J.S., Squire J., Thunnissen E., Ladanyi M. college of American pathologists international association for the study of lung cancer and association for molecular pathology. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the college of American pathologists, international association for the study of lung cancer, and association for molecular pathology. J. Mol. Diagn. 2013 Jul;15(4):415–453. - PubMed
-
- Rubio-Perez C. In silico prescription of anticancer drugs to cohorts of 28 tumor types reveals targeting opportunities. Canc. Cell. 2015;27(3):382–396. - PubMed
-
- Tafe L.J., Gorlov I.P., de Abreu F.B., Lefferts J.A., Liu X., Pettus J.R., Marotti J.D., Bloch K.J., Memoli V.A., Suriawinata A.A., Dragnev K.H., Fadul C.E., Schwartz G.N., Morgan C.R., Holderness B.M., Peterson J.D., Tsongalis G.J., Miller T.W., Chamberlin M.D. Implementation of a molecular tumor board: the impact on treatment decisions for 35 patients evaluated at dartmouth-hitchcock medical center. Oncol. 2015 Sep;20(9):1011–1018. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous