Ste2 receptor-mediated chemotropism of Fusarium graminearum contributes to its pathogenicity against wheat
- PMID: 32612109
- PMCID: PMC7329813
- DOI: 10.1038/s41598-020-67597-z
Ste2 receptor-mediated chemotropism of Fusarium graminearum contributes to its pathogenicity against wheat
Abstract
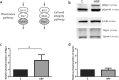
Fusarium Head Blight of wheat, caused by the filamentous fungus Fusarium graminearum, leads to devastating global food shortages and economic losses. While many studies have addressed the responses of both wheat and F. graminearum during their interaction, the possibility of fungal chemotropic sensing enabling pathogenicity remains unexplored. Based on recent findings linking the pheromone-sensing G-protein-coupled receptor Ste2 to host-directed chemotropism in Fusarium oxysporum, we investigated the role of the Ste2 receptor and its downstream signaling pathways in mediating chemotropism of F. graminearum. Interestingly, a chemotropic response of growing hyphae towards catalytically active Triticum aestivum 'Roblin' cultivar secreted peroxidases was detected, with deletion of STE2 in F. graminearum leading to loss of the observed response. At the same time, deletion of STE2 significantly decreased infection on germinating wheat coleoptiles, highlighting an association between Ste2, chemotropism and infection by F. graminearum. Further characterization revealed that the peroxidase-directed chemotropism is associated with stimulation of the fungal cell wall integrity mitogen-activated protein kinase signaling cascade. Altogether, this study demonstrates conservation of Ste2-mediated chemotropism by Fusarium species, and its important role in mediating pathogenicity.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Fusarium graminearum Ste3 G-Protein Coupled Receptor: A Mediator of Hyphal Chemotropism and Pathogenesis.mSphere. 2022 Dec 21;7(6):e0045622. doi: 10.1128/msphere.00456-22. Epub 2022 Nov 15. mSphere. 2022. PMID: 36377914 Free PMC article.
-
Autocrine pheromone signalling regulates community behaviour in the fungal pathogen Fusarium oxysporum.Nat Microbiol. 2019 Sep;4(9):1443-1449. doi: 10.1038/s41564-019-0456-z. Epub 2019 May 27. Nat Microbiol. 2019. PMID: 31133754
-
A peroxidase-derived ligand that induces Fusarium graminearum Ste2 receptor-dependent chemotropism.Front Cell Infect Microbiol. 2024 Jan 4;13:1287418. doi: 10.3389/fcimb.2023.1287418. eCollection 2023. Front Cell Infect Microbiol. 2024. PMID: 38239502 Free PMC article.
-
Contribution of cell wall degrading enzymes to pathogenesis of Fusarium graminearum: a review.J Basic Microbiol. 2009 Jun;49(3):231-41. doi: 10.1002/jobm.200800231. J Basic Microbiol. 2009. PMID: 19025875 Review.
-
Surfactin inhibits Fusarium graminearum by accumulating intracellular ROS and inducing apoptosis mechanisms.World J Microbiol Biotechnol. 2023 Oct 12;39(12):340. doi: 10.1007/s11274-023-03790-2. World J Microbiol Biotechnol. 2023. PMID: 37821760 Review.
Cited by
-
Conserved perception of host and non-host signals via the a-pheromone receptor Ste3 in Colletotrichum graminicola.Front Fungal Biol. 2024 Oct 7;5:1454633. doi: 10.3389/ffunb.2024.1454633. eCollection 2024. Front Fungal Biol. 2024. PMID: 39435183 Free PMC article.
-
Epistatic Relationship between MGV1 and TRI6 in the Regulation of Biosynthetic Gene Clusters in Fusarium graminearum.J Fungi (Basel). 2023 Aug 2;9(8):816. doi: 10.3390/jof9080816. J Fungi (Basel). 2023. PMID: 37623587 Free PMC article.
-
Update on the Basic Understanding of Fusarium graminearum Virulence Factors in Common Wheat Research.Plants (Basel). 2024 Apr 22;13(8):1159. doi: 10.3390/plants13081159. Plants (Basel). 2024. PMID: 38674569 Free PMC article. Review.
-
Hiding in plain sight: Genome-wide recombination and a dynamic accessory genome drive diversity in Fusarium oxysporum f.sp. ciceris.Proc Natl Acad Sci U S A. 2023 Jul 4;120(27):e2220570120. doi: 10.1073/pnas.2220570120. Epub 2023 Jun 26. Proc Natl Acad Sci U S A. 2023. PMID: 37364097 Free PMC article.
-
Kinesin-8-specific loop-2 controls the dual activities of the motor domain according to tubulin protofilament shape.Nat Commun. 2022 Jul 20;13(1):4198. doi: 10.1038/s41467-022-31794-3. Nat Commun. 2022. PMID: 35859148 Free PMC article.
References
-
- Gemma JN, Koske RE. Pre-infection interactions between roots and the mycorrhizal fungus Gigaspora gigantea: Chemotropism of germ-tubes and root growth response. Trans. Br. Mycol. Soc. 1988;91:123–132. doi: 10.1016/S0007-1536(88)80013-5. - DOI
-
- Jansson H-B, Johansson T, Nordbring-Hertz B, Tunlid A, Odham G. Chemotropic growth of germ-tubes of Cochliobolus sativus to barley roots or root exudates. Trans. Br. Mycol. Soc. 1988;90:647–650. doi: 10.1016/S0007-1536(88)80072-X. - DOI
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

