Ykt6-dependent endosomal recycling is required for Wnt secretion in the Drosophila wing epithelium
- PMID: 32611603
- PMCID: PMC7438013
- DOI: 10.1242/dev.185421
Ykt6-dependent endosomal recycling is required for Wnt secretion in the Drosophila wing epithelium
Abstract
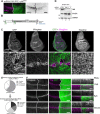
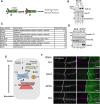
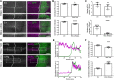
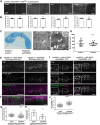
Morphogens are important signalling molecules for tissue development and their secretion requires tight regulation. In the wing imaginal disc of flies, the morphogen Wnt/Wingless is apically presented by the secreting cell and re-internalized before final long-range secretion. Why Wnt molecules undergo these trafficking steps and the nature of the regulatory control within the endosomal compartment remain unclear. Here, we have investigated how Wnts are sorted at the level of endosomes by the versatile v-SNARE Ykt6. Using in vivo genetics, proximity-dependent proteomics and in vitro biochemical analyses, we show that most Ykt6 is present in the cytosol, but can be recruited to de-acidified compartments and recycle Wnts to the plasma membrane via Rab4-positive recycling endosomes. Thus, we propose a molecular mechanism by which producing cells integrate and leverage endocytosis and recycling via Ykt6 to coordinate extracellular Wnt levels.
Keywords: Endosomal sorting; Morphogen trafficking; Wnt secretion; Wnt signalling.
© 2020. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures
Similar articles
-
Godzilla-dependent transcytosis promotes Wingless signalling in Drosophila wing imaginal discs.Nat Cell Biol. 2016 Apr;18(4):451-7. doi: 10.1038/ncb3325. Epub 2016 Mar 14. Nat Cell Biol. 2016. PMID: 26974662 Free PMC article.
-
The kinesin motor Klp98A mediates apical to basal Wg transport.Development. 2020 Aug 14;147(15):dev186833. doi: 10.1242/dev.186833. Development. 2020. PMID: 32665246 Free PMC article.
-
Active Wnt proteins are secreted on exosomes.Nat Cell Biol. 2012 Oct;14(10):1036-45. doi: 10.1038/ncb2574. Epub 2012 Sep 16. Nat Cell Biol. 2012. PMID: 22983114
-
Formation and maintenance of morphogen gradients: an essential role for the endomembrane system in Drosophila melanogaster wing development.Fly (Austin). 2011 Jul-Sep;5(3):266-71. doi: 10.4161/fly.5.3.16542. Epub 2011 Jul 1. Fly (Austin). 2011. PMID: 21654212 Review.
-
Integration of morphogen signalling within the growth regulatory network.Curr Opin Cell Biol. 2012 Apr;24(2):166-72. doi: 10.1016/j.ceb.2011.12.010. Epub 2012 Jan 16. Curr Opin Cell Biol. 2012. PMID: 22257639 Review.
Cited by
-
Microscopic and biochemical monitoring of endosomal trafficking and extracellular vesicle secretion in an endogenous in vivo model.J Extracell Vesicles. 2022 Sep;11(9):e12263. doi: 10.1002/jev2.12263. J Extracell Vesicles. 2022. PMID: 36103151 Free PMC article.
-
ULK1-mediated phosphorylation regulates the conserved role of YKT6 in autophagy.J Cell Sci. 2023 Feb 1;136(3):jcs260546. doi: 10.1242/jcs.260546. Epub 2023 Feb 9. J Cell Sci. 2023. PMID: 36644903 Free PMC article.
-
Phosphorylation of Ykt6 SNARE Domain Regulates Its Membrane Recruitment and Activity.Biomolecules. 2020 Nov 16;10(11):1560. doi: 10.3390/biom10111560. Biomolecules. 2020. PMID: 33207719 Free PMC article.
-
Visualization and Quantitation of Wg trafficking in the Drosophila Wing Imaginal Epithelium.Bio Protoc. 2021 Jun 5;11(11):e4040. doi: 10.21769/BioProtoc.4040. eCollection 2021 Jun 5. Bio Protoc. 2021. PMID: 34250206 Free PMC article.
-
Soluble Frizzled-related proteins promote exosome-mediated Wnt re-secretion.Commun Biol. 2024 Mar 1;7(1):254. doi: 10.1038/s42003-024-05881-8. Commun Biol. 2024. PMID: 38429359 Free PMC article.
References
-
- Baeg G. H., Lin X., Khare N., Baumgartner S. and Perrimon N. (2001). Heparan sulfate proteoglycans are critical for the organization of the extracellular distribution of Wingless. Development 128, 87-94. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

