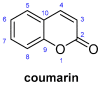
An Overview of Coumarin as a Versatile and Readily Accessible Scaffold with Broad-Ranging Biological Activities
- PMID: 32610556
- PMCID: PMC7370201
- DOI: 10.3390/ijms21134618
An Overview of Coumarin as a Versatile and Readily Accessible Scaffold with Broad-Ranging Biological Activities
Abstract
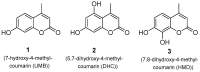
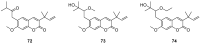
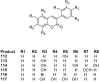
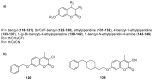
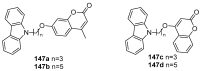
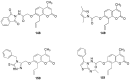
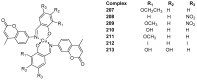
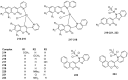
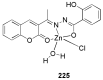
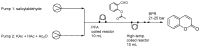
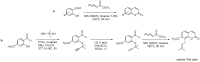
Privileged structures have been widely used as an effective template for the research and discovery of high value chemicals. Coumarin is a simple scaffold widespread in Nature and it can be found in a considerable number of plants as well as in some fungi and bacteria. In the last years, these natural compounds have been gaining an increasing attention from the scientific community for their wide range of biological activities, mainly due to their ability to interact with diverse enzymes and receptors in living organisms. In addition, coumarin nucleus has proved to be easily synthetized and decorated, giving the possibility of designing new coumarin-based compounds and investigating their potential in the treatment of various diseases. The versatility of coumarin scaffold finds applications not only in medicinal chemistry but also in the agrochemical field as well as in the cosmetic and fragrances industry. This review is intended to be a critical overview on coumarins, comprehensive of natural sources, metabolites, biological evaluations and synthetic approaches.
Keywords: biological activity; coumarins; metabolites; natural sources; synthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
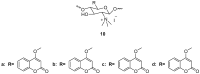
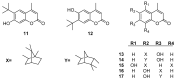
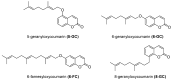
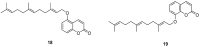
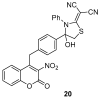
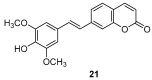
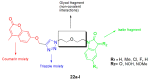
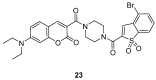
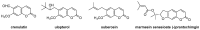


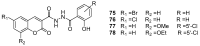
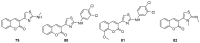
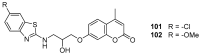
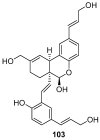
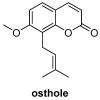
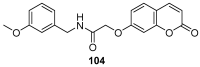
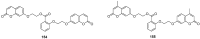

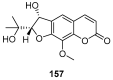
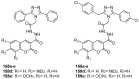
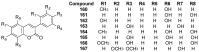

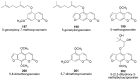
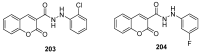
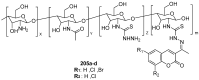
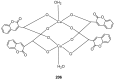
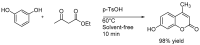
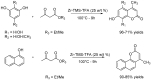
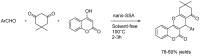
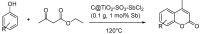
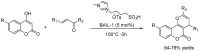
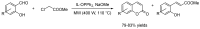
Similar articles
-
Coumarin Compounds in Medicinal Chemistry: Some Important Examples from the Last Years.Curr Top Med Chem. 2018;18(2):124-148. doi: 10.2174/1568026618666180329115523. Curr Top Med Chem. 2018. PMID: 29595110 Review.
-
Medicinal chemistry aspects and synthetic strategies of coumarin as aromatase inhibitors: an overview.Med Oncol. 2022 Dec 5;40(1):41. doi: 10.1007/s12032-022-01916-4. Med Oncol. 2022. PMID: 36471176 Review.
-
An Update on Synthesis of Coumarin Sulfonamides as Enzyme Inhibitors and Anticancer Agents.Molecules. 2022 Feb 28;27(5):1604. doi: 10.3390/molecules27051604. Molecules. 2022. PMID: 35268704 Free PMC article. Review.
-
Synthetic and natural coumarins as potent anticonvulsant agents: A review with structure-activity relationship.J Clin Pharm Ther. 2022 Jul;47(7):915-931. doi: 10.1111/jcpt.13644. Epub 2022 Mar 15. J Clin Pharm Ther. 2022. PMID: 35288962 Review.
-
Current developments of coumarin compounds in medicinal chemistry.Curr Pharm Des. 2013;19(21):3884-930. doi: 10.2174/1381612811319210013. Curr Pharm Des. 2013. PMID: 23438968 Review.
Cited by
-
Efficient Synthesis of Fluorescent Coumarins and Phosphorous-Containing Coumarin-Type Heterocycles via Palladium Catalyzed Cross-Coupling Reactions.Molecules. 2022 Nov 7;27(21):7649. doi: 10.3390/molecules27217649. Molecules. 2022. PMID: 36364471 Free PMC article.
-
Antimicrobial Activity of Lactones.Antibiotics (Basel). 2022 Sep 29;11(10):1327. doi: 10.3390/antibiotics11101327. Antibiotics (Basel). 2022. PMID: 36289985 Free PMC article. Review.
-
Coumarin: A natural solution for alleviating inflammatory disorders.Curr Res Pharmacol Drug Discov. 2024 Sep 25;7:100202. doi: 10.1016/j.crphar.2024.100202. eCollection 2024. Curr Res Pharmacol Drug Discov. 2024. PMID: 39398983 Free PMC article. Review.
-
Determination of Five Coumarins in Angelicae Pubescentis Radix from Different Origins by HPTLC-Scanning.J Anal Methods Chem. 2022 Aug 29;2022:3415938. doi: 10.1155/2022/3415938. eCollection 2022. J Anal Methods Chem. 2022. PMID: 36072919 Free PMC article.
-
Advances in biosynthesis of scopoletin.Microb Cell Fact. 2022 Aug 2;21(1):152. doi: 10.1186/s12934-022-01865-7. Microb Cell Fact. 2022. PMID: 35918699 Free PMC article. Review.
References
-
- Barot K.P., Jain S.V., Kremer L., Singh S., Ghate M.D. Recent advances and therapeutic journey of coumarins: Current status and perspectives. Med. Chem. Res. 2015;24:2771–2798. doi: 10.1007/s00044-015-1350-8. - DOI
-
- IUPAC . Nomenclature of Organic Chemistry. Pergamon Press; Oxford, UK: 1979.

