Coinhibition of activated p38 MAPKα and mTORC1 potentiates stemness maintenance of HSCs from SR1-expanded human cord blood CD34+ cells via inhibition of senescence
- PMID: 32602209
- PMCID: PMC7695631
- DOI: 10.1002/sctm.20-0129
Coinhibition of activated p38 MAPKα and mTORC1 potentiates stemness maintenance of HSCs from SR1-expanded human cord blood CD34+ cells via inhibition of senescence
Abstract
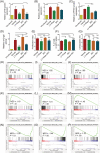
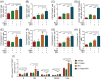
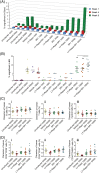
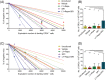
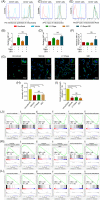
The stemness of ex vivo expanded hematopoietic stem cells (HSCs) is usually compromised by current methods. To explore the failure mechanism of stemness maintenance of human HSCs, which were expanded from human umbilical cord blood (hUCB) CD34+ cells, by differentiation inhibitor Stem Regenin 1 (SR1), an antagonist of aryl hydrocarbon receptor, we investigated the activity of p38 mitogen-activated protein kinase α (p38 MAPKα, p38α) and mammalian target of rapamycin complex 1 (mTORC1), and their effect on SR1-expanded hUCB CD34+ cells. Our results showed that cellular senescence occurred in the SR1-expanded hUCB CD34+ cells in which p38α and mTORC1 were successively activated. Furthermore, their coinhibition resulted in a further decrease in hUCB CD34+ cell senescence without an effect on apoptosis, promoted the maintenance of expanded phenotypic HSCs without differentiation inhibition, increased the hematopoietic reconstitution ability of multiple lineages, and potentiated the long-term self-renewal capability of HSCs from SR1-expanded hUCB CD34+ cells in NOD/Shi-scid/IL-2Rγnull mice. Our mechanistic study revealed that senescence inhibition by our strategy was mainly attributed to downregulation of the splicesome, proteasome formation, and pyrimidine metabolism signaling pathways. These results suggest that coinhibition of activated p38α and mTORC1 potentiates stemness maintenance of HSCs from SR1-expanded hUCB CD34+ cells via senescence inhibition. Thus, we established a new strategy to maintain the stemness of ex vivo differentiation inhibitor-expanded human HSCs via coinhibition of multiple independent senescence initiating signal pathways. This senescence inhibition-induced stemness maintenance of ex vivo expanded HSCs could also have an important role in other HSC expansion systems.
Keywords: HSC stemness maintenance; Stem Regenin 1; cellular senescence; ex vivo expansion; human cord blood CD34+ cells; mammalian target of rapamycin complex 1; p38 mitogen-activated protein kinase α.
© 2020 The Authors. STEM CELLS TRANSLATIONAL MEDICINE published by Wiley Periodicals LLC on behalf of AlphaMed Press.
Conflict of interest statement
The authors declared no potential conflicts of interest.
Figures
Similar articles
-
Inhibition of p38 MAPK activity promotes ex vivo expansion of human cord blood hematopoietic stem cells.Ann Hematol. 2012 Jun;91(6):813-23. doi: 10.1007/s00277-011-1397-7. Epub 2012 Jan 19. Ann Hematol. 2012. PMID: 22258328 Free PMC article.
-
Rapamycin enhances long-term hematopoietic reconstitution of ex vivo expanded mouse hematopoietic stem cells by inhibiting senescence.Transplantation. 2014 Jan 15;97(1):20-9. doi: 10.1097/TP.0b013e3182a7fcf8. Transplantation. 2014. PMID: 24092377 Free PMC article.
-
Low oxygen tension favored expansion and hematopoietic reconstitution of CD34(+) CD38(-) cells expanded from human cord blood-derived CD34(+) Cells.Biotechnol J. 2016 Jul;11(7):945-53. doi: 10.1002/biot.201500497. Epub 2016 Apr 29. Biotechnol J. 2016. PMID: 26997358
-
[Updated human hematopoietic stem cell findings: purification of human hematopoietic stem cells and elucidation of their hierarchy].Rinsho Ketsueki. 2018;59(10):1861-1871. doi: 10.11406/rinketsu.59.1861. Rinsho Ketsueki. 2018. PMID: 30305486 Review. Japanese.
-
Update on preclinical and clinical efforts on ex-vivo expansion of hematopoietic stem and progenitor cells.Curr Opin Hematol. 2022 Jul 1;29(4):167-173. doi: 10.1097/MOH.0000000000000714. Epub 2022 Feb 25. Curr Opin Hematol. 2022. PMID: 35220322 Review.
Cited by
-
Clinical Progress and Preclinical Insights Into Umbilical Cord Blood Transplantation Improvement.Stem Cells Transl Med. 2022 Sep 21;11(9):912-926. doi: 10.1093/stcltm/szac056. Stem Cells Transl Med. 2022. PMID: 35972332 Free PMC article. Review.
-
Human serum albumin promotes self-renewal and expansion of umbilical cord blood CD34+ hematopoietic stem/progenitor cells.Ann Transl Med. 2023 Jan 31;11(2):62. doi: 10.21037/atm-22-6383. Epub 2023 Jan 13. Ann Transl Med. 2023. PMID: 36819590 Free PMC article.
-
Time-series analysis of hematopoietic stem cells.Medicine (Baltimore). 2024 Feb 23;103(8):e36509. doi: 10.1097/MD.0000000000036509. Medicine (Baltimore). 2024. PMID: 38394540 Free PMC article.
-
DNA barcode to trace the development and differentiation of cord blood stem cells (Review).Mol Med Rep. 2021 Dec;24(6):849. doi: 10.3892/mmr.2021.12489. Epub 2021 Oct 13. Mol Med Rep. 2021. PMID: 34643250 Free PMC article. Review.
References
-
- Baron F, Ruggeri A, Nagler A. Methods of ex vivo expansion of human cord blood cells: challenges, successes and clinical implications. Expert Rev Hematol. 2016;9(3):297‐314. - PubMed
-
- Xie J, Zhang C. Ex vivo expansion of hematopoietic stem cells. Sci China Life Sci. 2015;58(9):839‐853. - PubMed
-
- Boelens JJ. The power of cord blood cells. Blood. 2016;127(26):3302‐3303. - PubMed
-
- Doan PL, Chute JP. Ex vivo expansion of murine and human hematopoietic stem cells. Methods Mol Biol. 2014;1185:211‐221. - PubMed
-
- Waskow C. Maintaining what is already there: strategies to rectify HSC transplantation dilemmas. Cell Stem Cell. 2015;17(3):258‐259. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

