Role of the Guanine Nucleotide Exchange Factor GBF1 in the Replication of RNA Viruses
- PMID: 32599855
- PMCID: PMC7354614
- DOI: 10.3390/v12060682
Role of the Guanine Nucleotide Exchange Factor GBF1 in the Replication of RNA Viruses
Abstract
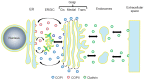
The guanine nucleotide exchange factor GBF1 is a well-known factor that can activate different ADP-ribosylation factor (Arf) proteins during the regulation of different cellular vesicular transport processes. In the last decade, it has become increasingly evident that GBF1 can also regulate different steps of the replication cycle of RNA viruses belonging to different virus families. GBF1 has been shown not only to facilitate the intracellular traffic of different viral and cellular elements during infection, but also to modulate the replication of viral RNA, the formation and maturation of viral replication complexes, and the processing of viral proteins through mechanisms that do not depend on its canonical role in intracellular transport. Here, we review the various roles that GBF1 plays during the replication of different RNA viruses.
Keywords: GBF1; RNA viruses; vesicle transport.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

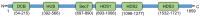
Similar articles
-
Enterovirus Infection Induces Massive Recruitment of All Isoforms of Small Cellular Arf GTPases to the Replication Organelles.J Virol. 2020 Dec 22;95(2):e01629-20. doi: 10.1128/JVI.01629-20. Print 2020 Dec 22. J Virol. 2020. PMID: 33087467 Free PMC article.
-
Investigation of the role of GBF1 in the replication of positive-sense single-stranded RNA viruses.J Gen Virol. 2018 Aug;99(8):1086-1096. doi: 10.1099/jgv.0.001099. Epub 2018 Jun 20. J Gen Virol. 2018. PMID: 29923822
-
A Redundant Mechanism of Recruitment Underlies the Remarkable Plasticity of the Requirement of Poliovirus Replication for the Cellular ArfGEF GBF1.J Virol. 2019 Oct 15;93(21):e00856-19. doi: 10.1128/JVI.00856-19. Print 2019 Nov 1. J Virol. 2019. PMID: 31375590 Free PMC article.
-
(+) RNA virus replication compartments: a safe home for (most) viral replication.Curr Opin Microbiol. 2016 Aug;32:82-88. doi: 10.1016/j.mib.2016.05.003. Epub 2016 May 30. Curr Opin Microbiol. 2016. PMID: 27253151 Free PMC article. Review.
-
Regulating the large Sec7 ARF guanine nucleotide exchange factors: the when, where and how of activation.Cell Mol Life Sci. 2014 Sep;71(18):3419-38. doi: 10.1007/s00018-014-1602-7. Epub 2014 Apr 13. Cell Mol Life Sci. 2014. PMID: 24728583 Free PMC article. Review.
Cited by
-
The Regulatory Role of GBF1 on Osteoclast Activation Through EIF2a Mediated ER Stress and Novel Marker FAM129A Induction.Front Cell Dev Biol. 2021 Aug 25;9:706768. doi: 10.3389/fcell.2021.706768. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34513838 Free PMC article.
-
Direct-Acting Antivirals and Host-Targeting Approaches against Enterovirus B Infections: Recent Advances.Pharmaceuticals (Basel). 2023 Jan 29;16(2):203. doi: 10.3390/ph16020203. Pharmaceuticals (Basel). 2023. PMID: 37259352 Free PMC article. Review.
-
Viral use and subversion of membrane organization and trafficking.J Cell Sci. 2021 Mar 4;134(5):jcs252676. doi: 10.1242/jcs.252676. J Cell Sci. 2021. PMID: 33664154 Free PMC article. Review.
-
Behavioral innovation and genomic novelty are associated with the exploitation of a challenging dietary opportunity by an avivorous bat.iScience. 2022 Aug 17;25(9):104973. doi: 10.1016/j.isci.2022.104973. eCollection 2022 Sep 16. iScience. 2022. PMID: 36093062 Free PMC article.
-
Treading a HOSTile path: Mapping the dynamic landscape of host cell-rotavirus interactions to explore novel host-directed curative dimensions.Virulence. 2021 Dec;12(1):1022-1062. doi: 10.1080/21505594.2021.1903198. Virulence. 2021. PMID: 33818275 Free PMC article. Review.
References
-
- Roberts K., Johnson A., Lewis J., Walter P., Raff M., Alberts B. Intracellular Vesicular Traffic. In: Alberts B., Johnson A., Lewis J., Morgan D., Raff M., Roberts K., Walter P., editors. Molecular Biology of the Cell. 6th ed. Garland Science; New York, NY, USA: 2019. pp. 749–812.
-
- Manolea F., Claude A., Chun J., Rosas J., Melanc P. Distinct functions for Arf guanine nucleotide exchange factors at the Golgi complex: GBF1 and BIGs are required for assembly and maintenance of the Golgi Stack and Trans- Golgi network, Respectively. Mol. Biol. Cell. 2008;19:523–535. doi: 10.1091/mbc.e07-04-0394. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

