Reprogramming of profibrotic macrophages for treatment of bleomycin-induced pulmonary fibrosis
- PMID: 32597014
- PMCID: PMC7411553
- DOI: 10.15252/emmm.202012034
Reprogramming of profibrotic macrophages for treatment of bleomycin-induced pulmonary fibrosis
Abstract
Fibrotic diseases cause organ failure that lead to ~45% of all deaths in the United States. Activated macrophages stimulate fibrosis by secreting cytokines that induce fibroblasts to synthesize collagen and extracellular matrix proteins. Although suppression of macrophage-derived cytokine production can halt progression of fibrosis, therapeutic agents that prevent release of these cytokines (e.g., TLR7 agonists) have proven too toxic to administer systemically. Based on the expression of folate receptor β solely on activated myeloid cells, we have created a folate-targeted TLR7 agonist (FA-TLR7-54) that selectively accumulates in profibrotic macrophages and suppresses fibrosis-inducing cytokine production. We demonstrate that FA-TLR7-54 reprograms M2-like fibrosis-inducing macrophages into fibrosis-suppressing macrophages, resulting in dramatic declines in profibrotic cytokine release, hydroxyproline biosynthesis, and collagen deposition, with concomitant increases in alveolar airspaces. Although nontargeted TLR7-54 is lethal at fibrosis-suppressing doses, FA-TLR7-54 halts fibrosis without evidence of toxicity. Taken together, FA-TLR7-54 is shown to constitute a novel and potent approach for treating fibrosis without causing dose-limiting systemic toxicities.
Keywords: bleomycin; folate receptor β; idiopathic pulmonary fibrosis; macrophages; toll-like receptor 7.
© 2020 The Authors. Published under the terms of the CC BY 4.0 license.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
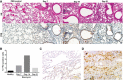
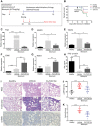
- A
Mice with BLM‐induced experimental fibrosis were stained using a monoclonal antibody to mouse FRβ (F3). Representative FRβ‐positive macrophages are marked with red arrows. H&E and FRβ IHC staining were performed on days 7, 14, and 21 post‐BLM‐induced lung injury. More than 90 × 106 cells were quantified per section using Aperio Image Scope (Leica Biosystems). Scale bars, 100 μm.
- B
Quantification of FRβ staining in sections from panel A.
- C, D
IHC staining of healthy (C) or IPF (D) human lung tissue with a monoclonal antibody to human FRβ (m909). Scale bars, 200 μm.
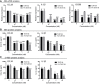
- A–C
Human monocytic (THP‐1) cells were induced to acquire an M2‐like phenotype (see Materials and Methods) and treated with different concentrations of TLR7‐54 or FA‐TLR7‐54 for either 48 h (A–B) or 2 h (C). In the latter case, after the 2 h incubation, culture medium was replaced with drug‐free medium and incubation was continued for 46 h. All treatment groups were then analyzed by qPCR for gene expression, and cell supernatants were analyzed for secreted cytokines by ELISA. (A) Changes in mRNA levels of indicated profibrotic macrophage markers by induced by different concentrations of TLR7‐54 and FA‐TLR7‐54 (n = 3, technical replicates). (B‐C) Changes in CCL18 and IL‐1β in the culture media induced upon treatment with TLR7‐54 and FA‐TLR7‐54 for the treatment regimens (n = 3, technical replicates).
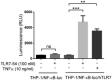
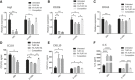
- A–F
M2‐induced human monocyte‐derived macrophages were treated with 100 nM of the indicated drug either continuously for 48 h, or initially for 2 h in the presence or absence of FA‐glucosamine (competition) followed by 46 h in the absence of drug (2+46 h), as described in Fig 1. mRNA levels of profibrotic markers, Arg1 (A), CD206 (B), and CD163 (C), and protein levels of secreted profibrotic CCL18 (D) and anti‐fibrotic cytokines, CXCL10 (E) and IL‐6 (F) (n = 3, technical replicates), were then determined. Changes in both sets of cytokines were inhibited by blockade of unoccupied folate receptors with excess FA‐glucosamine (2+46 h, competition).
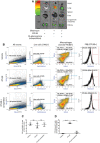
- A
Healthy mice or BLM‐induced mice were tail vein injected on day 10 with 10 nmol OTL38 in the absence or presence of 200‐fold excess FA‐glucosamine to block all folate receptors. After 2 h, mice were euthanized, resected, and imaged for fluorescence intensity (n = 5).
- B
Alternatively, lungs from mice injected with 100 nmol OTL38 in the presence or absence of 200‐fold excess FA‐glucosamine were collagenase digested and stained with 7‐AAD plus antibodies to CD11b and F4/80 prior to FACS analysis (n = 3). Representative plots showing the gating strategy yielding live macrophages (7‐AAD− CD11b+ F4/80+) and OTL38‐positive macrophages are shown.
- C
Percentages of live macrophages in BLM‐induced mice (n = 3).
- D
Percentages of lung macrophages that accumulated OTL38 in vivo (n = 3).
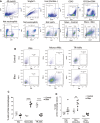
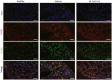
- A
Representative plots showing the gating strategy leading to various macrophage subpopulations.
- B
Representative plots showing FRβ expression on interstitial macrophages (IMs), monocyte‐derived alveolar macrophages (Mono‐AMs), and tissue‐resident alveolar macrophages (TR‐AMs).
- C
Percentages of IMs, Mono‐AMs, and TR‐AMs present in the total macrophage pool (Ly6C‐ gate) (n = 5–7).
- D
Proportion of FRβ‐expressing IMs, FRβ‐expressing Mono‐AMs, and FRβ‐expressing TR‐AMs in the corresponding parent populations (n = 5–7). All samples were derived at the same time and processed in parallel.
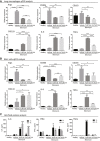
- A–C
Healthy mice or BLM‐induced mice were injected intravenously on day 10 with either vehicle (3% DMSO in PBS), or 10 nmol TLR7‐54 or FA‐TLR7‐54 dissolved in vehicle, and 1 or 4 h later sacrificed to collect both lungs and bronchoalveolar lavage fluid (BALF). (A) Lungs were digested, and macrophages were isolated by flow cytometry prior to analysis for expression of the indicated mRNAs by qPCR (n = 3). (B) BALF cells were pelleted and similarly analyzed for the indicated mRNAs (n = 3). (C) BALF supernatant was also collected and analyzed by ELISA for IL‐6, IFNα, and TNFα (n = 3).
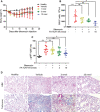
- A
BLM‐induced mice were treated every other day beginning on day 10 with 10 nmol/dose FA‐TLR7‐54 or TLR7‐54.
- B
Survival analysis of the treatment groups (healthy, n = 5; others, n = 10).
- C–H
BALF was collected on day 21 and centrifuged to isolate cell pellets. Cells (primarily macrophages) were analyzed by qPCR for Arg1 (C), MMP9 (D), TIMP3 (E), CD86 (F), and IRAK4 (G) (n = 5). BALF supernatant was analyzed by ELISA for IFNγ (H) (n = 5).
- I–K
Lungs were resected and subjected to hematoxylin–eosin (H&E), trichome (collagen), and α‐SMA IHC staining (scale bars, 200 μm), (J) or hydrolyzed and analyzed for hydroxyproline content as a measure of collagen content (healthy, n = 5; others, n = 10), (K) Ashcroft score quantitation of fibrosis (n = 5).
- A
Analysis of body weight change versus time (n = 10).
- B
Analysis of the number of cells per milliliter of BALF (n = 5).
- C
Quantitation of total hydroxyproline content per right lung (healthy control, n = 5; others, n = 7–9).
- D
H&E staining and trichrome staining of lung tissue (scale bars, 200 μm).
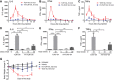
- A–C
Measurement of plasma IL‐6 (A), IFNα (B), and TNFα (C) (n = 3).
- D–F
Effect of drug concentration on plasma levels of IL‐6 (D), IFNα (E), and TNFα (F) at 1.5 h, 1 h, or 1 h after treatment, respectively (n = 2).
- G
Change in body weight as a measure of systemic toxicity during alternate‐day dosing (n = 2).
Similar articles
-
Effect of an immunotoxin to folate receptor beta on bleomycin-induced experimental pulmonary fibrosis.Clin Exp Immunol. 2010 Aug;161(2):348-56. doi: 10.1111/j.1365-2249.2010.04182.x. Epub 2010 Jun 9. Clin Exp Immunol. 2010. PMID: 20550546 Free PMC article.
-
Icariside Ⅱ attenuates bleomycin-induced pulmonary fibrosis by modulating macrophage polarization.J Ethnopharmacol. 2023 Dec 5;317:116810. doi: 10.1016/j.jep.2023.116810. Epub 2023 Jun 16. J Ethnopharmacol. 2023. PMID: 37331450
-
Tumor necrosis factor-α accelerates the resolution of established pulmonary fibrosis in mice by targeting profibrotic lung macrophages.Am J Respir Cell Mol Biol. 2014 Apr;50(4):825-37. doi: 10.1165/rcmb.2013-0386OC. Am J Respir Cell Mol Biol. 2014. PMID: 24325577 Free PMC article.
-
The novel inhibitor PRI-724 for Wnt/β-catenin/CBP signaling ameliorates bleomycin-induced pulmonary fibrosis in mice.Exp Lung Res. 2019 Sep;45(7):188-199. doi: 10.1080/01902148.2019.1638466. Epub 2019 Jul 12. Exp Lung Res. 2019. PMID: 31298961
-
Lung specific homing of diphenyleneiodonium chloride improves pulmonary fibrosis by inhibiting macrophage M2 metabolic program.J Adv Res. 2023 Feb;44:213-225. doi: 10.1016/j.jare.2022.04.012. Epub 2022 Apr 29. J Adv Res. 2023. PMID: 36725191 Free PMC article.
Cited by
-
Tumor-specific activation of folate receptor beta enables reprogramming of immune cells in the tumor microenvironment.Front Immunol. 2024 Feb 7;15:1354735. doi: 10.3389/fimmu.2024.1354735. eCollection 2024. Front Immunol. 2024. PMID: 38384467 Free PMC article.
-
Injectable demineralized bone matrix particles and their hydrogel bone grafts loaded with β-tricalcium phosphate powder and granules: A comparative study.Mater Today Bio. 2022 Sep 9;16:100422. doi: 10.1016/j.mtbio.2022.100422. eCollection 2022 Dec. Mater Today Bio. 2022. PMID: 36133794 Free PMC article.
-
Properties of Pleural Mesothelial Cells in Idiopathic Pulmonary Fibrosis and Cryptogenic Organizing Pneumonia.J Korean Med Sci. 2023 Aug 7;38(31):e242. doi: 10.3346/jkms.2023.38.e242. J Korean Med Sci. 2023. PMID: 37550810 Free PMC article.
-
Mitochondrial folate pathway regulates myofibroblast differentiation and silica-induced pulmonary fibrosis.J Transl Med. 2023 Jun 6;21(1):365. doi: 10.1186/s12967-023-04241-0. J Transl Med. 2023. PMID: 37280614 Free PMC article.
-
Macrophages and HLA-Class II Alleles in Multiple Sclerosis: Insights in Therapeutic Dynamics.Int J Mol Sci. 2024 Jul 4;25(13):7354. doi: 10.3390/ijms25137354. Int J Mol Sci. 2024. PMID: 39000461 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

