Mounting evidence of FKBP12 implication in neurodegeneration
- PMID: 32594030
- PMCID: PMC7749462
- DOI: 10.4103/1673-5374.284980
Mounting evidence of FKBP12 implication in neurodegeneration
Abstract
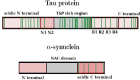
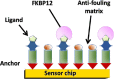
Intrinsically disordered proteins, such as tau or α-synuclein, have long been associated with a dysfunctional role in neurodegenerative diseases. In Alzheimer's and Parkinson's' diseases, these proteins, sharing a common chemical-physical pattern with alternating hydrophobic and hydrophilic domains rich in prolines, abnormally aggregate in tangles in the brain leading to progressive loss of neurons. In this review, we present an overview linking the studies on the implication of the peptidyl-prolyl isomerase domain of immunophilins, and notably FKBP12, to a variety of neurodegenerative diseases, focusing on the molecular origin of such a role. The involvement of FKBP12 dysregulation in the aberrant aggregation of disordered proteins pinpoints this protein as a possible therapeutic target and, at the same time, as a predictive biomarker for early diagnosis in neurodegeneration, calling for the development of reliable, fast and cost-effective detection methods in body fluids for community-based screening campaigns.
Keywords: Alzheimer’s disease; FK506 binding protein; FKBP12; Parkinson’s disease; biomarker; detections; neurodegeneration; tau protein; α-synuclein.
Conflict of interest statement
None
Figures


Similar articles
-
Peptidyl-prolyl isomerase activity of FK506 binding protein 12 prevents tau peptide from aggregating.Protein Eng Des Sel. 2013 Sep;26(9):539-46. doi: 10.1093/protein/gzt033. Epub 2013 Jul 5. Protein Eng Des Sel. 2013. PMID: 23832849
-
FKBP12-immunopositive inclusions in patients with α-synucleinopathies.Brain Res. 2018 Feb 1;1680:39-45. doi: 10.1016/j.brainres.2017.12.012. Epub 2017 Dec 12. Brain Res. 2018. PMID: 29246765
-
Unraveling the role of peptidyl-prolyl isomerases in neurodegeneration.Mol Neurobiol. 2011 Aug;44(1):13-27. doi: 10.1007/s12035-011-8184-2. Epub 2011 May 7. Mol Neurobiol. 2011. PMID: 21553017 Review.
-
Comparative analysis of different peptidyl-prolyl isomerases reveals FK506-binding protein 12 as the most potent enhancer of alpha-synuclein aggregation.J Biol Chem. 2011 Jul 29;286(30):26687-701. doi: 10.1074/jbc.M110.182303. Epub 2011 Jun 7. J Biol Chem. 2011. PMID: 21652707 Free PMC article.
-
Tau and Alpha Synuclein Synergistic Effect in Neurodegenerative Diseases: When the Periphery Is the Core.Int J Mol Sci. 2020 Jul 16;21(14):5030. doi: 10.3390/ijms21145030. Int J Mol Sci. 2020. PMID: 32708732 Free PMC article. Review.
Cited by
-
Oxidative-Signaling in Neural Stem Cell-Mediated Plasticity: Implications for Neurodegenerative Diseases.Antioxidants (Basel). 2021 Jul 6;10(7):1088. doi: 10.3390/antiox10071088. Antioxidants (Basel). 2021. PMID: 34356321 Free PMC article. Review.
-
Circulating proteomic biomarkers for diagnosing sporadic amyotrophic lateral sclerosis: a cross-sectional study.Neural Regen Res. 2024 Aug 1;19(8):1842-1848. doi: 10.4103/1673-5374.389357. Epub 2023 Nov 8. Neural Regen Res. 2024. PMID: 38103252 Free PMC article.
-
Comprehensive Quantitative Proteome Analysis of Aedes aegypti Identifies Proteins and Pathways Involved in Wolbachia pipientis and Zika Virus Interference Phenomenon.Front Physiol. 2021 Feb 25;12:642237. doi: 10.3389/fphys.2021.642237. eCollection 2021. Front Physiol. 2021. PMID: 33716790 Free PMC article.
-
An arrayed genome-wide perturbation screen identifies the ribonucleoprotein Hnrnpk as rate-limiting for prion propagation.EMBO J. 2022 Dec 1;41(23):e112338. doi: 10.15252/embj.2022112338. Epub 2022 Oct 18. EMBO J. 2022. PMID: 36254605 Free PMC article.
-
Proteomic Analysis of Rhesus Macaque Brain Explants Treated With Borrelia burgdorferi Identifies Host GAP-43 as a Potential Factor Associated With Lyme Neuroborreliosis.Front Cell Infect Microbiol. 2021 Jun 10;11:647662. doi: 10.3389/fcimb.2021.647662. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34178719 Free PMC article.
References
-
- Avramut M, Achim CL. Immunophilins and their ligands: insights into survival and growth of human neurons. Physiol Behav. 2002;77:463–468. - PubMed
-
- Blackburn EA, Walkinshaw MD. Targeting FKBP isoforms with small-molecule ligands. Curr Opin Pharmacol. 2011;11:365–371. - PubMed
-
- Bonner JM, Boulianne GL. Diverse structures, functions and uses of FK506 binding proteins. Cell Signal. 2017;38:97–105. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

