Priming the Proteasome to Protect against Proteotoxicity
- PMID: 32589934
- PMCID: PMC7321925
- DOI: 10.1016/j.molmed.2020.02.007
Priming the Proteasome to Protect against Proteotoxicity
Abstract
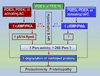
Increased proteotoxic stress (IPTS) resulting from the increased production or decreased removal of abnormally folded proteins is recognized as an important pathogenic factor for a large group of highly disabling and life-threatening human diseases, such as neurodegenerative disorders and many heart diseases. The proteasome is pivotal to the timely removal of abnormal proteins but its functional capacity often becomes inadequate in the disease conditions; consequently, proteasome functional insufficiency in return exacerbates IPTS. Recent research in proteasome biology reveals that the proteasome can be activated by endogenous protein kinases, making it possible to pharmacologically prime the proteasome for treating diseases with IPTS.
Keywords: cAMP-dependent protein kinase; cGMP-dependent protein kinase; desmin-related cardiomyopathy; phosphodiesterases; proteasome; proteotoxicity.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
Proteotoxicity: an underappreciated pathology in cardiac disease.J Mol Cell Cardiol. 2014 Jun;71:3-10. doi: 10.1016/j.yjmcc.2013.12.015. Epub 2013 Dec 28. J Mol Cell Cardiol. 2014. PMID: 24380730 Free PMC article. Review.
-
p62 Stages an interplay between the ubiquitin-proteasome system and autophagy in the heart of defense against proteotoxic stress.Trends Cardiovasc Med. 2011 Nov;21(8):224-8. doi: 10.1016/j.tcm.2012.05.015. Trends Cardiovasc Med. 2011. PMID: 22902070 Free PMC article. Review.
-
PDE1 inhibition facilitates proteasomal degradation of misfolded proteins and protects against cardiac proteinopathy.Sci Adv. 2019 May 22;5(5):eaaw5870. doi: 10.1126/sciadv.aaw5870. eCollection 2019 May. Sci Adv. 2019. PMID: 31131329 Free PMC article.
-
Protein kinase g positively regulates proteasome-mediated degradation of misfolded proteins.Circulation. 2013 Jul 23;128(4):365-76. doi: 10.1161/CIRCULATIONAHA.113.001971. Epub 2013 Jun 14. Circulation. 2013. PMID: 23770744 Free PMC article.
-
Autophagy and polyglutamine diseases.Prog Neurobiol. 2012 May;97(2):67-82. doi: 10.1016/j.pneurobio.2011.08.013. Epub 2011 Sep 10. Prog Neurobiol. 2012. PMID: 21930185 Free PMC article. Review.
Cited by
-
Role of Proteostasis Regulation in the Turnover of Stress Granules.Int J Mol Sci. 2022 Nov 23;23(23):14565. doi: 10.3390/ijms232314565. Int J Mol Sci. 2022. PMID: 36498892 Free PMC article. Review.
-
The Heart of the Alzheimer's: A Mindful View of Heart Disease.Front Physiol. 2021 Jan 27;11:625974. doi: 10.3389/fphys.2020.625974. eCollection 2020. Front Physiol. 2021. PMID: 33584340 Free PMC article.
-
Editorial: Proteostasis in cardiac health and disease.Front Mol Biosci. 2024 May 31;11:1433721. doi: 10.3389/fmolb.2024.1433721. eCollection 2024. Front Mol Biosci. 2024. PMID: 38882607 Free PMC article. No abstract available.
-
Overexpression of Nfe2l1 increases proteasome activity and delays vision loss in a preclinical model of human blindness.Sci Adv. 2023 Jul 14;9(28):eadd5479. doi: 10.1126/sciadv.add5479. Epub 2023 Jul 14. Sci Adv. 2023. PMID: 37450596 Free PMC article.
-
S14-Phosphorylated RPN6 Mediates Proteasome Activation by PKA and Alleviates Proteinopathy.Circ Res. 2023 Sep 15;133(7):572-587. doi: 10.1161/CIRCRESAHA.123.322887. Epub 2023 Aug 29. Circ Res. 2023. PMID: 37641975 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

