Immobilization rapidly selects for chemoresistant ovarian cancer cells with enhanced ability to enter dormancy
- PMID: 32589792
- PMCID: PMC8505013
- DOI: 10.1002/bit.27479
Immobilization rapidly selects for chemoresistant ovarian cancer cells with enhanced ability to enter dormancy
Abstract
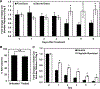
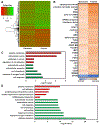
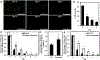
Around 20-30% of ovarian cancer patients exhibit chemoresistance, but there are currently no methods to predict whether a patient will respond to chemotherapy. Here, we discovered that chemoresistant ovarian cancer cells exhibit enhanced survival in a quiescent state upon experiencing the stress of physical confinement. When immobilized in stiff silica gels, most ovarian cancer cells die within days, but surviving cells exhibit hallmarks of single-cell dormancy. Upon extraction from gels, the cells resume proliferation but demonstrate enhanced viability upon reimmobilization, indicating that initial immobilization selects for cells with a higher propensity to enter dormancy. RNA-seq analysis of the extracted cells shows they have signaling responses similar to cells surviving cisplatin treatment, and in comparison to chemoresistant patient cohorts, they share differentially expressed genes that are associated with platinum-resistance pathways. Furthermore, these extracted cells demonstrate greater resistance to cisplatin and paclitaxel, despite being proliferative. In contrast, serum starvation and hypoxia could not effectively select for chemoresistant cells upon removal of the environmental stress. These findings demonstrate that ovarian cancer chemoresistance and the ability to enter dormancy are linked, and immobilization rapidly distinguishes chemoresistant cells. This platform could be suitable for mechanistic studies, drug development, or as a clinical diagnostic tool.
Keywords: chemoresistance; immobilization; ovarian cancer; quiescence; silica gel.
© 2020 Wiley Periodicals LLC.
Conflict of interest statement
Conflict of Interest Statement
The authors declare that there are no conflicts of interests.
Figures

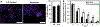

Similar articles
-
Differential activation of NF-κB signaling is associated with platinum and taxane resistance in MyD88 deficient epithelial ovarian cancer cells.Int J Biochem Cell Biol. 2015 Apr;61:90-102. doi: 10.1016/j.biocel.2015.02.001. Epub 2015 Feb 11. Int J Biochem Cell Biol. 2015. PMID: 25681684
-
HE4 promotes collateral resistance to cisplatin and paclitaxel in ovarian cancer cells.J Ovarian Res. 2016 May 17;9(1):28. doi: 10.1186/s13048-016-0240-0. J Ovarian Res. 2016. PMID: 27184254 Free PMC article.
-
Time-staggered inhibition of JNK effectively sensitizes chemoresistant ovarian cancer cells to cisplatin and paclitaxel.Oncol Rep. 2016 Jan;35(1):593-601. doi: 10.3892/or.2015.4377. Epub 2015 Nov 2. Oncol Rep. 2016. PMID: 26534836
-
Chemoresistance in Ovarian Cancer: Prospects for New Drugs.Anticancer Agents Med Chem. 2021;21(6):668-678. doi: 10.2174/1871520620666200908104835. Anticancer Agents Med Chem. 2021. PMID: 32900355 Review.
-
Targeting Mitochondria for Treatment of Chemoresistant Ovarian Cancer.Int J Mol Sci. 2019 Jan 8;20(1):229. doi: 10.3390/ijms20010229. Int J Mol Sci. 2019. PMID: 30626133 Free PMC article. Review.
Cited by
-
The oxidative aging model integrated various risk factors in type 2 diabetes mellitus at system level.Front Endocrinol (Lausanne). 2023 May 24;14:1196293. doi: 10.3389/fendo.2023.1196293. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37293508 Free PMC article.
-
The Influence of Ligand Density and Degradability on Hydrogel Induced Breast Cancer Dormancy and Reactivation.Adv Healthc Mater. 2021 Jun;10(11):e2002227. doi: 10.1002/adhm.202002227. Epub 2021 Apr 30. Adv Healthc Mater. 2021. PMID: 33929776 Free PMC article.
-
Colorectal cancer and dormant metastases: Put to sleep or destroy?World J Gastrointest Oncol. 2024 Jun 15;16(6):2304-2317. doi: 10.4251/wjgo.v16.i6.2304. World J Gastrointest Oncol. 2024. PMID: 38994146 Free PMC article.
-
Pancreatic Ductal Adenocarcinoma Cortical Mechanics and Clinical Implications.Front Oncol. 2022 Jan 31;12:809179. doi: 10.3389/fonc.2022.809179. eCollection 2022. Front Oncol. 2022. PMID: 35174086 Free PMC article. Review.
References
-
- Abubaker K, Latifi A, Chan E, Luwor RB, Burns CJ, Thompson EW, … Ahmed N (2015). Enhanced activation of STAT3 in ascites-derived recurrent ovarian tumors: inhibition of cisplatin-induced STAT3 activation reduced tumorigenicity of ovarian cancer by a loss of cancer stem cell-like characteristics. Journal of Cancer Stem Cell Research.
-
- Aguirre-ghiso JA, Estrada Y, Liu D, & Ossowski L (2003). ERK MAPK Activity as a Determinant of Tumor Growth and Dormancy ; Regulation by p38 SAPK. Cancer Research, 63(7), 1684–1695. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

