Drug Loading in Poly(butyl cyanoacrylate)-Based Polymeric Microbubbles
- PMID: 32589435
- PMCID: PMC7611892
- DOI: 10.1021/acs.molpharmaceut.0c00242
Drug Loading in Poly(butyl cyanoacrylate)-Based Polymeric Microbubbles
Abstract
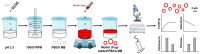
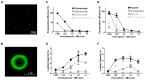
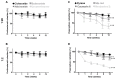
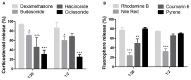
Microbubbles (MB) are routinely used ultrasound (US) contrast agents that have recently attracted increasing attention as stimuli-responsive drug delivery systems. To better understand MB-based drug delivery, we studied the role of drug hydrophobicity and molecular weight on MB loading, shelf-life stability, US properties, and drug release. Eight model drugs, varying in hydrophobicity and molecular weight, were loaded into the shell of poly(butyl cyanoacrylate) (PBCA) MB. In the case of drugs with progesterone as a common structural backbone (i.e., for corticosteroids), loading capacity and drug release correlated well with hydrophobicity and molecular weight. Conversely, when employing drugs with no structural similarity (i.e., four different fluorescent dyes), loading capacity and release did not correlate with hydrophobicity and molecular weight. All model drug-loaded MB formulations could be equally efficiently destroyed upon exposure to US. Together, these findings provide valuable insights on how the physicochemical properties of (model) drug molecules affect their loading and retention in and US-induced release from polymeric MB, thereby facilitating the development of drug-loaded MB formulations for US-triggered drug delivery.
Keywords: PBCA; corticosteroids; drug delivery; microbubbles; theranostics; ultrasound.
Conflict of interest statement
FK and TL hold a patent on multimodal US imaging using PBCA-based polymeric microbubbles.
Figures




Similar articles
-
Physicochemical Characterization of the Shell Composition of PBCA-Based Polymeric Microbubbles.Macromol Biosci. 2017 Oct;17(10). doi: 10.1002/mabi.201700002. Epub 2017 Mar 29. Macromol Biosci. 2017. PMID: 28371270
-
PBCA-based polymeric microbubbles for molecular imaging and drug delivery.J Control Release. 2017 Aug 10;259:128-135. doi: 10.1016/j.jconrel.2017.03.006. Epub 2017 Mar 6. J Control Release. 2017. PMID: 28279799 Free PMC article.
-
Enhanced Stable Cavitation and Nonlinear Acoustic Properties of Poly(butyl cyanoacrylate) Polymeric Microbubbles after Bioconjugation.ACS Biomater Sci Eng. 2024 Jan 8;10(1):75-81. doi: 10.1021/acsbiomaterials.2c01021. Epub 2022 Oct 31. ACS Biomater Sci Eng. 2024. PMID: 36315422
-
Polymeric microbubbles for ultrasonic molecular imaging and targeted therapeutics.J Biomater Sci Polym Ed. 2011;22(4-6):417-28. doi: 10.1163/092050610X540440. J Biomater Sci Polym Ed. 2011. PMID: 21144258 Review.
-
Drug-Loaded Microbubbles Combined with Ultrasound for Thrombolysis and Malignant Tumor Therapy.Biomed Res Int. 2019 Oct 1;2019:6792465. doi: 10.1155/2019/6792465. eCollection 2019. Biomed Res Int. 2019. PMID: 31662987 Free PMC article. Review.
Cited by
-
Analyzing the Mechanical Properties of Free-Standing PACA Thin Films Using Microindentation Technique.Polymers (Basel). 2022 Nov 11;14(22):4863. doi: 10.3390/polym14224863. Polymers (Basel). 2022. PMID: 36432991 Free PMC article.
-
Ultrasonically Enhanced ZD2767P-Carboxypeptidase G2 Deactivates Cisplatin-Resistant Human Lung Cancer Cells.Oxid Med Cell Longev. 2022 Nov 4;2022:9191233. doi: 10.1155/2022/9191233. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 36388164 Free PMC article.
-
Aminolysis-mediated single-step surface functionalization of poly (butyl cyanoacrylate) microbubbles for ultrasound molecular imaging.J Nanobiotechnology. 2024 Sep 1;22(1):528. doi: 10.1186/s12951-024-02806-9. J Nanobiotechnology. 2024. PMID: 39218888 Free PMC article.
-
Nonspherical ultrasound microbubbles.Proc Natl Acad Sci U S A. 2023 Mar 28;120(13):e2218847120. doi: 10.1073/pnas.2218847120. Epub 2023 Mar 20. Proc Natl Acad Sci U S A. 2023. PMID: 36940339 Free PMC article.
-
Targeted Microbubbles for Drug, Gene, and Cell Delivery in Therapy and Immunotherapy.Pharmaceutics. 2023 May 30;15(6):1625. doi: 10.3390/pharmaceutics15061625. Pharmaceutics. 2023. PMID: 37376072 Free PMC article. Review.
References
-
- Lindner JR. Microbubbles in medical imaging: current applications and future directions. Nat Rev Drug Discov. 2004;3:527–532. - PubMed
-
- Klibanov AL. Ligand-carrying gas-filled microbubbles: ultrasound contrast agents for targeted molecular imaging, Bioconjug. Chem. 2005;16:9–17. - PubMed
-
- Kiessling F, Huppert J, Palmowski M. Functional and molecular ultrasound imaging: concepts and contrast agents. Curr Med Chem. 2009;16:627–642. - PubMed
-
- Unger EC, Hersh E, Vannan M, Matsunaga TO, McCreery T. Local drug and gene delivery through microbubbles. Prog Cardiovasc Dis. 2001;44:45–54. - PubMed
-
- Mitragotri S. Healing sound: the use of ultrasound in drug delivery and other therapeutic applications. Nat Rev Drug Discov. 2005;4:255–260. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

