Recovery of ovarian function by human embryonic stem cell-derived mesenchymal stem cells in cisplatin-induced premature ovarian failure in mice
- PMID: 32586410
- PMCID: PMC7318510
- DOI: 10.1186/s13287-020-01769-6
Recovery of ovarian function by human embryonic stem cell-derived mesenchymal stem cells in cisplatin-induced premature ovarian failure in mice
Abstract
Background: Clinical use of mesenchymal stem cells (MSCs) requires a uniform cell population, and their harvesting is invasive and produces a limited number of cells. Human embryonic stem cell-derived MSCs (hESC-MSCs) can differentiate into three germ layers and possess immunosuppressive effects in vitro. Anticancer treatment is a well-known risk factor for premature ovarian failure (POF). In this study, we investigated the effect of hESC-MSC on recovery of ovarian function in cisplatin-induced POF in mice.
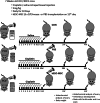
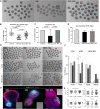
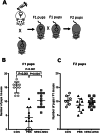
Methods: Female mice received intraperitoneal cisplatin for 10 days. On day 12, CHA15-derived hESC-MSCs were transplanted into the mice by tail vein injection. An injection of PBS served as the negative control. Ovaries were removed 28 days after transplantation for assessment of ovarian histology, immunostaining, and fertility testing by superovulation and in vitro fertilization. hESC-MSC transplantation into mice with cisplatin-induced damage restored body weight and ovary size.
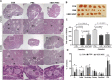
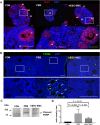
Results: Mean primary and primordial follicle counts in the hESC-MSC group were significantly improved compared to the PBS group (P < 0.05), and counts of zona pellucida remnants, an apoptotic sign in ovarian follicles, were significantly reduced (P < 0.05). TUNEL assays and cleaved PARP immunostaining indicated apoptosis, which led to loss of ovarian stromal cells in negative control mice, while Ki-67 was higher in the hESC-MSC group and in non-cisplatin-treated controls than in the PBS group. Ovulation was reduced in the PBS group but recovered significantly in the hESC-MSC group. Rates of blastocyst formation from ovulated eggs and live births per mouse also recovered significantly in the hESC-MSC group.
Conclusions: hESC-MSC restored structure and function in the cisplatin-damaged ovary. Our study provides new insights into the great clinical potential of human hESC-MSC in treating POF.
Keywords: Chemotherapy-induced premature ovarian failure; Human embryonic stem cell-derived MSC; Ovarian stromal cell apoptosis; Recovery of ovarian function.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Human Umbilical Cord Mesenchymal Stem Cells Improve Ovarian Function in Chemotherapy-Induced Premature Ovarian Failure Mice Through Inhibiting Apoptosis and Inflammation via a Paracrine Mechanism.Reprod Sci. 2021 Jun;28(6):1718-1732. doi: 10.1007/s43032-021-00499-1. Epub 2021 Mar 9. Reprod Sci. 2021. PMID: 33751459
-
Effects of single and multiple transplantations of human umbilical cord mesenchymal stem cells on the recovery of ovarian function in the treatment of premature ovarian failure in mice.J Ovarian Res. 2021 Sep 15;14(1):119. doi: 10.1186/s13048-021-00871-4. J Ovarian Res. 2021. PMID: 34526090 Free PMC article.
-
Clusterin-carrying extracellular vesicles derived from human umbilical cord mesenchymal stem cells restore the ovarian function of premature ovarian failure mice through activating the PI3K/AKT pathway.Stem Cell Res Ther. 2024 Sep 13;15(1):300. doi: 10.1186/s13287-024-03926-7. Stem Cell Res Ther. 2024. PMID: 39272156 Free PMC article.
-
Transplantation of human umbilical cord mesenchymal stem cells to treat premature ovarian failure.Stem Cell Res Ther. 2021 Aug 11;12(1):454. doi: 10.1186/s13287-021-02529-w. Stem Cell Res Ther. 2021. PMID: 34380572 Free PMC article. Review.
-
Intraovarian injection of autologous human mesenchymal stem cells increases estrogen production and reduces menopausal symptoms in women with premature ovarian failure: two case reports and a review of the literature.J Med Case Rep. 2020 Jul 18;14(1):108. doi: 10.1186/s13256-020-02426-5. J Med Case Rep. 2020. PMID: 32680541 Free PMC article. Review.
Cited by
-
Cellular and molecular mechanisms of highly active mesenchymal stem cells in the treatment of senescence of rhesus monkey ovary.Stem Cell Res Ther. 2024 Jan 8;15(1):14. doi: 10.1186/s13287-023-03631-x. Stem Cell Res Ther. 2024. PMID: 38191526 Free PMC article.
-
Paeoniflorin Alleviates Cisplatin-Induced Diminished Ovarian Reserve by Restoring the Function of Ovarian Granulosa Cells via Activating FSHR/cAMP/PKA/CREB Signaling Pathway.Molecules. 2023 Dec 15;28(24):8123. doi: 10.3390/molecules28248123. Molecules. 2023. PMID: 38138611 Free PMC article.
-
Mesenchymal stem cells: ideal seeds for treating diseases.Hum Cell. 2021 Nov;34(6):1585-1600. doi: 10.1007/s13577-021-00578-0. Epub 2021 Jul 16. Hum Cell. 2021. PMID: 34272720 Free PMC article. Review.
-
Endometrial stem cell-derived exosomes repair cisplatin-induced premature ovarian failure via Hippo signaling pathway.Heliyon. 2024 May 21;10(10):e31639. doi: 10.1016/j.heliyon.2024.e31639. eCollection 2024 May 30. Heliyon. 2024. PMID: 38831834 Free PMC article.
-
Application of mesenchymal stem cells derived from human pluripotent stem cells in regenerative medicine.World J Stem Cells. 2021 Dec 26;13(12):1826-1844. doi: 10.4252/wjsc.v13.i12.1826. World J Stem Cells. 2021. PMID: 35069985 Free PMC article. Review.
References
-
- de Vos FY, Nuver J, Willemse PH, van der Zee AG, Messerschmidt J, Burgerhof JG, et al. Long-term survivors of ovarian malignancies after cisplatin-based chemotherapy; cardiovascular risk factors and signs of vascular damage. Eur J Cancer. 2004;40(5):696–700. doi: 10.1016/j.ejca.2003.11.026. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

