LRP-1 Promotes Colon Cancer Cell Proliferation in 3D Collagen Matrices by Mediating DDR1 Endocytosis
- PMID: 32582700
- PMCID: PMC7283560
- DOI: 10.3389/fcell.2020.00412
LRP-1 Promotes Colon Cancer Cell Proliferation in 3D Collagen Matrices by Mediating DDR1 Endocytosis
Abstract
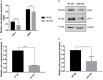
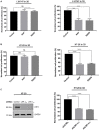
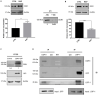
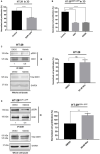
Low density lipoprotein receptor related protein-1 (LRP-1) is a large ubiquitous endocytic receptor mediating the clearance of various molecules from the extracellular matrix. Several studies have shown that LRP-1 plays crucial roles during tumorigenesis functioning as a main signal pathway regulator, especially by interacting with other cell-surface receptors. Discoïdin Domain Receptors (DDRs), type I collagen receptors with tyrosine kinase activity, have previously been associated with tumor invasion and aggressiveness in diverse tumor environments. Here, we addressed whether it could exist functional interplays between LRP-1 and DDR1 to control colon carcinoma cell behavior in three-dimensional (3D) collagen matrices. We found that LRP-1 established tight molecular connections with DDR1 at the plasma membrane in colon cancer cells. In this tumor context, we provide evidence that LRP-1 regulates by endocytosis the cell surface levels of DDR1 expression. The LRP-1 mediated endocytosis of DDR1 increased cell proliferation by promoting cell cycle progression into S phase and decreasing apoptosis. In this study, we identified a new molecular way that controls the cell-surface expression of DDR1 and consequently the colon carcinoma cell proliferation and apoptosis and highlighted an additional mechanism by which LRP-1 carries out its sensor activity of the tumor microenvironment.
Keywords: 3D collagen matrix; DDR1; LRP-1; colon cancer cell; proliferation.
Copyright © 2020 Le, Bennasroune, Collin, Hachet, Lehrter, Rioult, Dedieu, Morjani and Appert-Collin.
Figures
Similar articles
-
A membrane-type-1 matrix metalloproteinase (MT1-MMP)-discoidin domain receptor 1 axis regulates collagen-induced apoptosis in breast cancer cells.PLoS One. 2015 Mar 16;10(3):e0116006. doi: 10.1371/journal.pone.0116006. eCollection 2015. PLoS One. 2015. PMID: 25774665 Free PMC article.
-
DDR1 and MT1-MMP Expression Levels Are Determinant for Triggering BIK-Mediated Apoptosis by 3D Type I Collagen Matrix in Invasive Basal-Like Breast Carcinoma Cells.Front Pharmacol. 2019 May 3;10:462. doi: 10.3389/fphar.2019.00462. eCollection 2019. Front Pharmacol. 2019. PMID: 31130862 Free PMC article.
-
Age-related modifications of type I collagen impair DDR1-induced apoptosis in non-invasive breast carcinoma cells.Cell Adh Migr. 2018;12(4):335-347. doi: 10.1080/19336918.2018.1472182. Epub 2018 Jun 28. Cell Adh Migr. 2018. PMID: 29733741 Free PMC article.
-
Discoidin Domain Receptors: Potential Actors and Targets in Cancer.Front Pharmacol. 2016 Mar 14;7:55. doi: 10.3389/fphar.2016.00055. eCollection 2016. Front Pharmacol. 2016. PMID: 27014069 Free PMC article. Review.
-
Collagen and Discoidin Domain Receptor 1 Partnership: A Multifaceted Role in the Regulation of Breast Carcinoma Cell Phenotype.Front Cell Dev Biol. 2021 Dec 22;9:808625. doi: 10.3389/fcell.2021.808625. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 35004699 Free PMC article. Review.
Cited by
-
Salinomycin-Loaded High-Density Lipoprotein Exerts Promising Anti-Ovarian Cancer Effects by Inhibiting Epithelial-Mesenchymal Transition.Int J Nanomedicine. 2022 Sep 8;17:4059-4071. doi: 10.2147/IJN.S380598. eCollection 2022. Int J Nanomedicine. 2022. PMID: 36105618 Free PMC article.
-
The evolving landscape of PCSK9 inhibition in cancer.Eur J Pharmacol. 2023 Jun 15;949:175721. doi: 10.1016/j.ejphar.2023.175721. Epub 2023 Apr 12. Eur J Pharmacol. 2023. PMID: 37059376 Free PMC article. Review.
-
The Yin and Yang of Discoidin Domain Receptors (DDRs): Implications in Tumor Growth and Metastasis Development.Cancers (Basel). 2021 Apr 6;13(7):1725. doi: 10.3390/cancers13071725. Cancers (Basel). 2021. PMID: 33917302 Free PMC article. Review.
-
Discoidin domain receptors orchestrate cancer progression: A focus on cancer therapies.Cancer Sci. 2021 Mar;112(3):962-969. doi: 10.1111/cas.14789. Epub 2021 Jan 27. Cancer Sci. 2021. PMID: 33377205 Free PMC article. Review.
-
DDR1 functions as an immune negative factor in colorectal cancer by regulating tumor-infiltrating T cells through IL-18.Cancer Sci. 2022 Nov;113(11):3672-3685. doi: 10.1111/cas.15533. Epub 2022 Aug 24. Cancer Sci. 2022. PMID: 35969377 Free PMC article.
References
-
- Assent D., Bourgot I., Hennuy B., Geurts P., Noel A., Foidart J. M., et al. (2015). A membrane-type-1 matrix metalloproteinase (MT1-MMP)-discoidin domain receptor 1 axis regulates collagen-induced apoptosis in breast cancer cells. PLoS One 10:e0116006. 10.1371/journal.pone.0116006 - DOI - PMC - PubMed
-
- Beaujouin M., Prebois C., Derocq D., Laurent-Matha V., Masson O., Pattingre S., et al. (2010). Pro-cathepsin D interacts with the extracellular domain of the beta chain of LRP1 and promotes LRP1-dependent fibroblast outgrowth. J. Cell. Sci. 123(Pt 19) 3336–3346. 10.1242/jcs.070938 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

