Pro-angiogenic Activity Discriminates Human Adipose-Derived Stromal Cells From Retinal Pericytes: Considerations for Cell-Based Therapy of Diabetic Retinopathy
- PMID: 32582693
- PMCID: PMC7295949
- DOI: 10.3389/fcell.2020.00387
Pro-angiogenic Activity Discriminates Human Adipose-Derived Stromal Cells From Retinal Pericytes: Considerations for Cell-Based Therapy of Diabetic Retinopathy
Abstract
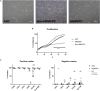
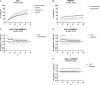
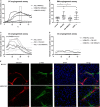
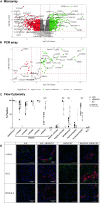
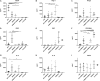
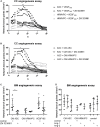
Diabetic retinopathy (DR) is a frequent diabetes-associated complication. Pericyte dropout can cause increased vascular permeability and contribute to vascular occlusion. Adipose-derived stromal cells (ASC) have been suggested to replace pericytes and restore microvascular support as potential therapy of DR. In models of DR, ASC not only generated a cytoprotective and reparative environment by the secretion of trophic factors but also engrafted and integrated into the retina in a pericyte-like fashion. The aim of this study was to compare the pro-angiogenic features of human ASC and human retinal microvascular pericytes (HRMVPC) in vitro. The proliferation and the expression of ASC and HRMVPC markers were compared. Adhesion to high glucose-conditioned endothelial extracellular matrix, mimicking the diabetic microenvironment, was measured. The angiogenesis-promoting features of both cell types and their conditioned media on human retinal endothelial cells (EC) were assessed. To identify a molecular basis for the observed differences, gene expression profiling was performed using whole-genome microarrays, and data were validated using PCR arrays and flow cytometry. Based on multiplex cytokine results, functional studies on selected growth factors were performed to assess their role in angiogenic support. Despite a distinct heterogeneity in ASC and HRMVPC cultures with an overlap of expressed markers, ASC differed functionally from HRMVPC. Most importantly, the pro-angiogenic activity was solely featured by ASC, whereas HRMVPC actively suppressed vascular network formation. HRMVPC, in contrast to ASC, showed impaired adhesion and proliferation on the high glucose-conditioned endothelial extracellular matrix. These data were supported by gene expression profiles with differentially expressed genes. The vessel-stabilizing factors were more highly expressed in HRMVPC, and the angiogenesis-promoting factors were more highly expressed in ASC. The vascular endothelial growth factor receptor-2 inhibition efficiently abolished the ASC angiogenic supportive capacities, whereas the addition of angiopoietin-1 and angiopoietin-2 did not alter these effects. Our results clearly show that ASC are pro-angiogenic, whereas HRMVPC are marked by anti-angiogenic/EC-stabilizing features. These data support ASC as pericyte replacement in DR but also suggest a careful risk-to-benefit analysis to take full advantage of the ASC therapeutic features.
Keywords: angiogenesis; angiopoietin; diabetic retinopathy; human adipose-derived stromal cells; human retinal pericytes; vascular–endothelial growth factor.
Copyright © 2020 Kremer, Gebauer, Elvers-Hornung, Uhlig, Hammes, Beltramo, Steeb, Harmsen, Sticht, Klueter, Bieback and Fiori.
Figures
Similar articles
-
The Pericytic Phenotype of Adipose Tissue-Derived Stromal Cells Is Promoted by NOTCH2.Stem Cells. 2018 Feb;36(2):240-251. doi: 10.1002/stem.2726. Epub 2017 Nov 8. Stem Cells. 2018. PMID: 29067740
-
Adipose-derived mesenchymal stromal cells reverse high glucose-induced reduction of angiogenesis in human retinal microvascular endothelial cells.Cytotherapy. 2020 May;22(5):261-275. doi: 10.1016/j.jcyt.2020.02.005. Epub 2020 Apr 1. Cytotherapy. 2020. PMID: 32247542
-
Research on the role of exosomes secreted by immortalized adipose-derived mesenchymal stem cells differentiated into pericytes in the repair of high glucose-induced retinal vascular endothelial cell damage.Exp Eye Res. 2024 Oct;247:110046. doi: 10.1016/j.exer.2024.110046. Epub 2024 Aug 14. Exp Eye Res. 2024. PMID: 39147191
-
[Aging and retinal vascular diseases].Nippon Ganka Gakkai Zasshi. 2007 Mar;111(3):207-30; discussion 231. Nippon Ganka Gakkai Zasshi. 2007. PMID: 17402563 Review. Japanese.
-
Importance of pericytes and mechanisms of pericyte loss during diabetes retinopathy.Diabetes Obes Metab. 2008 Jan;10(1):53-63. doi: 10.1111/j.1463-1326.2007.00795.x. Epub 2007 Oct 15. Diabetes Obes Metab. 2008. PMID: 17941874 Review.
Cited by
-
Pooled human bone marrow-derived mesenchymal stromal cells with defined trophic factors cargo promote dermal wound healing in diabetic rats by improved vascularization and dynamic recruitment of M2-like macrophages.Front Immunol. 2022 Aug 19;13:976511. doi: 10.3389/fimmu.2022.976511. eCollection 2022. Front Immunol. 2022. PMID: 36059533 Free PMC article.
-
Abnormalities of retinal function in type 2 diabetes mellitus patients without clinical diabetic retinopathy detected by multifocal electroretinogram.BMC Ophthalmol. 2024 Feb 15;24(1):71. doi: 10.1186/s12886-024-03335-7. BMC Ophthalmol. 2024. PMID: 38360630 Free PMC article.
-
A Review on Mesenchymal Stem Cells for Treatment of Retinal Diseases.Stem Cell Rev Rep. 2021 Aug;17(4):1154-1173. doi: 10.1007/s12015-020-10090-x. Epub 2021 Jan 6. Stem Cell Rev Rep. 2021. PMID: 33410097 Free PMC article. Review.
-
Multifocal Electroretinogram Can Detect the Abnormal Retinal Change in Early Stage of type2 DM Patients without Apparent Diabetic Retinopathy.J Diabetes Res. 2021 Feb 23;2021:6644691. doi: 10.1155/2021/6644691. eCollection 2021. J Diabetes Res. 2021. PMID: 33681384 Free PMC article.
-
Effects of High Glucose Concentration on Pericyte-Like Differentiated Human Adipose-Derived Mesenchymal Stem Cells.Int J Mol Sci. 2021 Apr 27;22(9):4604. doi: 10.3390/ijms22094604. Int J Mol Sci. 2021. PMID: 33925714 Free PMC article.
References
-
- Bieback K., Hecker A., Schlechter T., Hofmann I., Brousos N., Redmer T., et al. (2012). Replicative aging and differentiation potential of human adipose tissue-derived mesenchymal stromal cells expanded in pooled human or fetal bovine serum. Cytotherapy 14 570–583. 10.3109/14653249.2011.652809 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

