Time Course of Immune Response and Immunomodulation During Normal and Delayed Healing of Musculoskeletal Wounds
- PMID: 32582170
- PMCID: PMC7287024
- DOI: 10.3389/fimmu.2020.01056
Time Course of Immune Response and Immunomodulation During Normal and Delayed Healing of Musculoskeletal Wounds
Abstract
Single trauma injuries or isolated fractures are often manageable and generally heal without complications. In contrast, high-energy trauma results in multi/poly-trauma injury patterns presenting imbalanced pro- and anti- inflammatory responses often leading to immune dysfunction. These injuries often exhibit delayed healing, leading to fibrosis of injury sites and delayed healing of fractures depending on the intensity of the compounding traumas. Immune dysfunction is accompanied by a temporal shift in the innate and adaptive immune cells distribution, triggered by the overwhelming release of an arsenal of inflammatory mediators such as complements, cytokines and damage associated molecular patterns (DAMPs) from necrotic cells. Recent studies have implicated this dysregulated inflammation in the poor prognosis of polytraumatic injuries, however, interventions focusing on immunomodulating inflammatory cellular composition and activation, if administered incorrectly, can result in immune suppression and unintended outcomes. Immunomodulation therapy is promising but should be conducted with consideration for the spatial and temporal distribution of the immune cells during impaired healing. This review describes the current state of knowledge in the spatiotemporal distribution patterns of immune cells at various stages during musculoskeletal wound healing, with a focus on recent advances in the field of Osteoimmunology, a study of the interface between the immune and skeletal systems, in long bone fractures. The goals of this review are to (1) discuss wound and fracture healing processes of normal and delayed healing in skeletal muscles and long bones; (2) provide a balanced perspective on temporal distributions of immune cells and skeletal cells during healing; and (3) highlight recent therapeutic interventions used to improve fracture healing. This review is intended to promote an understanding of the importance of inflammation during normal and delayed wound and fracture healing. Knowledge gained will be instrumental in developing novel immunomodulatory approaches for impaired healing.
Keywords: delayed fracture healing; dysregulated inflammatory response; fracture healing; osteoimmunology; wound healing.
Copyright © 2020 Muire, Mangum and Wenke.
Figures
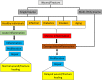

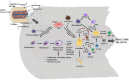

Similar articles
-
Impairment of early fracture healing by skeletal muscle trauma is restored by FK506.BMC Musculoskelet Disord. 2017 Jun 12;18(1):253. doi: 10.1186/s12891-017-1617-y. BMC Musculoskelet Disord. 2017. PMID: 28606129 Free PMC article.
-
Osteoimmunology of Fracture Healing.Curr Osteoporos Rep. 2024 Jun;22(3):330-339. doi: 10.1007/s11914-024-00869-z. Epub 2024 Apr 15. Curr Osteoporos Rep. 2024. PMID: 38616228 Free PMC article. Review.
-
Severe muscle trauma triggers heightened and prolonged local musculoskeletal inflammation and impairs adjacent tibia fracture healing.J Musculoskelet Neuronal Interact. 2016 Jun 1;16(2):122-34. J Musculoskelet Neuronal Interact. 2016. PMID: 27282456 Free PMC article.
-
Pleiotropic actions of Vitamin D in composite musculoskeletal trauma.Injury. 2020 Oct;51(10):2099-2109. doi: 10.1016/j.injury.2020.06.023. Epub 2020 Jun 18. Injury. 2020. PMID: 32624209 Review.
-
Advances in Immunomodulation and Immune Engineering Approaches to Improve Healing of Extremity Wounds.Int J Mol Sci. 2022 Apr 7;23(8):4074. doi: 10.3390/ijms23084074. Int J Mol Sci. 2022. PMID: 35456892 Free PMC article. Review.
Cited by
-
Crosstalk Between Innate and T Cell Adaptive Immunity With(in) the Muscle.Front Physiol. 2020 Sep 18;11:573347. doi: 10.3389/fphys.2020.573347. eCollection 2020. Front Physiol. 2020. PMID: 33071827 Free PMC article. Review.
-
Identification of miRNA Regulatory Networks and Candidate Markers for Fracture Healing in Mice.Comput Math Methods Med. 2021 Nov 16;2021:2866475. doi: 10.1155/2021/2866475. eCollection 2021. Comput Math Methods Med. 2021. PMID: 34840596 Free PMC article.
-
Blood transfusion practices affect CD4+ CD25+ FOXP3+ regulatory T cells/T helper-17 cells and the clinical outcome of geriatric patients with hip fracture.Aging (Albany NY). 2021 Sep 1;13(17):21408-21420. doi: 10.18632/aging.203479. Epub 2021 Sep 1. Aging (Albany NY). 2021. PMID: 34470917 Free PMC article.
-
Translating musculoskeletal bioengineering into tissue regeneration therapies.Sci Transl Med. 2022 Oct 12;14(666):eabn9074. doi: 10.1126/scitranslmed.abn9074. Epub 2022 Oct 12. Sci Transl Med. 2022. PMID: 36223445 Free PMC article. Review.
-
Febrile-Range Hyperthermia Can Prevent Toxic Effects of Neutrophil Extracellular Traps on Mesenchymal Stem Cells.Int J Mol Sci. 2022 Dec 19;23(24):16208. doi: 10.3390/ijms232416208. Int J Mol Sci. 2022. PMID: 36555846 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

