Targeting the ERG oncogene with splice-switching oligonucleotides as a novel therapeutic strategy in prostate cancer
- PMID: 32581342
- PMCID: PMC7493922
- DOI: 10.1038/s41416-020-0951-2
Targeting the ERG oncogene with splice-switching oligonucleotides as a novel therapeutic strategy in prostate cancer
Abstract
Background: The ERG oncogene, a member of the ETS family of transcription factor encoding genes, is a genetic driver of prostate cancer. It is activated through a fusion with the androgen-responsive TMPRSS2 promoter in 50% of cases. There is therefore significant interest in developing novel therapeutic agents that target ERG. We have taken an antisense approach and designed morpholino-based oligonucleotides that target ERG by inducing skipping of its constitutive exon 4.
Methods: We designed antisense morpholino oligonucleotides (splice-switching oligonucleotides, SSOs) that target both the 5' and 3' splice sites of ERG's exon 4. We tested their efficacy in terms of inducing exon 4 skipping in two ERG-positive cell lines, VCaP prostate cancer cells and MG63 osteosarcoma cells. We measured their effect on cell proliferation, migration and apoptosis. We also tested their effect on xenograft tumour growth in mice and on ERG protein expression in a human prostate cancer radical prostatectomy sample ex vivo.
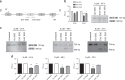
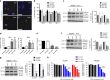
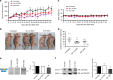
Results: In VCaP cells, both SSOs were effective at inducing exon 4 skipping, which resulted in a reduction of overall ERG protein levels up to 96 h following a single transfection. SSO-induced ERG reduction decreased cell proliferation, cell migration and significantly increased apoptosis. We observed a concomitant reduction in protein levels for cyclin D1, c-Myc and the Wnt signalling pathway member β-catenin as well as a marker of activated Wnt signalling, p-LRP6. We tested the 3' splice site SSO in MG63 xenografts in mice and observed a reduction in tumour growth. We also demonstrated that the 3' splice site SSO caused a reduction in ERG expression in a patient-derived prostate tumour tissue cultured ex vivo.
Conclusions: We have successfully designed and tested morpholino-based SSOs that cause a marked reduction in ERG expression, resulting in decreased cell proliferation, a reduced migratory phenotype and increased apoptosis. Our initial tests on mouse xenografts and a human prostate cancer radical prostatectomy specimen indicate that SSOs can be effective for oncogene targeting in vivo. As such, this study encourages further in vivo therapeutic studies using SSOs targeting the ERG oncogene.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
The Evolutionarily Conserved Cassette Exon 7b Drives ERG's Oncogenic Properties.Transl Oncol. 2019 Jan;12(1):134-142. doi: 10.1016/j.tranon.2018.09.001. Epub 2018 Oct 5. Transl Oncol. 2019. PMID: 30296658 Free PMC article.
-
Identification of a Small Molecule That Selectively Inhibits ERG-Positive Cancer Cell Growth.Cancer Res. 2018 Jul 1;78(13):3659-3671. doi: 10.1158/0008-5472.CAN-17-2949. Epub 2018 Apr 30. Cancer Res. 2018. PMID: 29712692
-
Role of dutasteride in pre-clinical ETS fusion-positive prostate cancer models.Prostate. 2012 Oct 1;72(14):1542-9. doi: 10.1002/pros.22509. Epub 2012 Mar 13. Prostate. 2012. PMID: 22415461
-
The Expression of Proto-Oncogene ETS-Related Gene (ERG) Plays a Central Role in the Oncogenic Mechanism Involved in the Development and Progression of Prostate Cancer.Int J Mol Sci. 2022 Apr 26;23(9):4772. doi: 10.3390/ijms23094772. Int J Mol Sci. 2022. PMID: 35563163 Free PMC article. Review.
-
The prognostic and predictive value of TMPRSS2-ERG gene fusion and ERG protein expression in prostate cancer biopsies.Dan Med J. 2016 Dec;63(12):B5319. Dan Med J. 2016. PMID: 27910803 Review.
Cited by
-
Splice-switch oligonucleotide-based combinatorial platform prioritizes synthetic lethal targets CHK1 and BRD4 against MYC-driven hepatocellular carcinoma.Bioeng Transl Med. 2022 Sep 3;8(1):e10363. doi: 10.1002/btm2.10363. eCollection 2023 Jan. Bioeng Transl Med. 2022. PMID: 36684069 Free PMC article.
-
Effects of Doxorubicin, Epirubicin, and Liposomal Doxorubicin (Anthracycline) on cardiac function in patients with osteosarcoma and their influencing factors.Clin Transl Oncol. 2024 Jun;26(6):1459-1466. doi: 10.1007/s12094-023-03372-6. Epub 2024 Feb 8. Clin Transl Oncol. 2024. PMID: 38329609
-
Poly(ADP-ribose) Polyremase-1 (PARP-1) Inhibition: A Promising Therapeutic Strategy for ETS-Expressing Tumours.Int J Mol Sci. 2023 Aug 30;24(17):13454. doi: 10.3390/ijms241713454. Int J Mol Sci. 2023. PMID: 37686260 Free PMC article. Review.
-
How Driver Oncogenes Shape and Are Shaped by Alternative Splicing Mechanisms in Tumors.Cancers (Basel). 2023 May 26;15(11):2918. doi: 10.3390/cancers15112918. Cancers (Basel). 2023. PMID: 37296881 Free PMC article. Review.
-
Steering research on mRNA splicing in cancer towards clinical translation.Nat Rev Cancer. 2024 Dec;24(12):887-905. doi: 10.1038/s41568-024-00750-2. Epub 2024 Oct 9. Nat Rev Cancer. 2024. PMID: 39384951 Free PMC article. Review.
References
-
- Werner MH, et al. The solution structure of the human ETS1-DNA complex reveals a novel mode of binding and true side chain intercalation. Cell. 1995;83:761–771. - PubMed
-
- Rao VN, Papas TS, Reddy E. ERG, a human ets-related gene on chromosome 21: alternative splicing, polyadenylation, and translation. Science. 1987;237:635–639. - PubMed
-
- Adamo P, Ladomery MR. The oncogene ERG: a key factor in prostate cancer. Oncogene. 2015;35:403–414. - PubMed
-
- Rosen P, Sesterhenn IA, Brassell SA, McLeod DG, Srivastava S, Dobi A. Clinical potential of the ERG oncoprotein in prostate cancer. Nat. Rev. Urol. 2012;10:483–487. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

