Muscarinic-dependent phosphorylation of the cardiac ryanodine receptor by protein kinase G is mediated by PI3K-AKT-nNOS signaling
- PMID: 32580946
- PMCID: PMC7450129
- DOI: 10.1074/jbc.RA120.014054
Muscarinic-dependent phosphorylation of the cardiac ryanodine receptor by protein kinase G is mediated by PI3K-AKT-nNOS signaling
Abstract
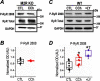
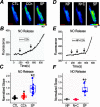
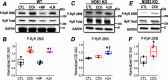
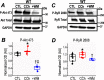
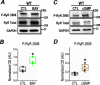
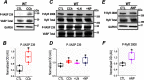
Post-translational modifications of proteins involved in calcium handling in myocytes, such as the cardiac ryanodine receptor (RyR2), critically regulate cardiac contractility. Recent studies have suggested that phosphorylation of RyR2 by protein kinase G (PKG) might contribute to the cardioprotective effects of cholinergic stimulation. However, the specific mechanisms underlying these effects remain unclear. Here, using murine ventricular myocytes, immunoblotting, proximity ligation as-says, and nitric oxide imaging, we report that phosphorylation of Ser-2808 in RyR2 induced by the muscarinic receptor agonist carbachol is mediated by a signaling axis comprising phosphoinositide 3-phosphate kinase, Akt Ser/Thr kinase, nitric oxide synthase 1, nitric oxide, soluble guanylate cyclase, cyclic GMP (cGMP), and PKG. We found that this signaling pathway is compartmentalized in myocytes, as it was distinct from atrial natriuretic peptide receptor-cGMP-PKG-RyR2 Ser-2808 signaling and independent of muscarinic-induced phosphorylation of Ser-239 in vasodilator-stimulated phosphoprotein. These results provide detailed insights into muscarinic-induced PKG signaling and the mediators that regulate cardiac RyR2 phosphorylation critical for cardiovascular function.
Keywords: AKT; AKT PKB; NOS1; NOS3; PI3K; cardiac RyR2; cyclase; guanylate cyclase (guanylyl cyclase); nitric oxide; nitric oxide synthase; protein kinase G (PKG); ryanodine receptor; soluble guanylate; vasodilator stimulatory phosphoprotein.
© 2020 Baine et al.
Conflict of interest statement
Conflict of interest—The authors declare that they have no conflicts of interest with the contents of this article.
Figures

Similar articles
-
Nitric oxide regulates AKT phosphorylation and nuclear translocation in cultured retinal cells.Cell Signal. 2013 Dec;25(12):2424-39. doi: 10.1016/j.cellsig.2013.08.001. Epub 2013 Aug 17. Cell Signal. 2013. PMID: 23958999
-
Potential contribution of ryanodine receptor 2 upregulation to cGMP/PKG signaling-induced cone degeneration in cyclic nucleotide-gated channel deficiency.FASEB J. 2020 May;34(5):6335-6350. doi: 10.1096/fj.201901951RR. Epub 2020 Mar 16. FASEB J. 2020. PMID: 32173907 Free PMC article.
-
Nitric oxide synthase and cyclic GMP signaling in cardiac myocytes: from contractility to remodeling.J Mol Cell Cardiol. 2012 Feb;52(2):330-40. doi: 10.1016/j.yjmcc.2011.07.029. Epub 2011 Aug 7. J Mol Cell Cardiol. 2012. PMID: 21843527 Review.
-
Single L-type Ca(2+) channel regulation by cGMP-dependent protein kinase type I in adult cardiomyocytes from PKG I transgenic mice.Cardiovasc Res. 2003 Nov 1;60(2):268-77. doi: 10.1016/s0008-6363(03)00546-7. Cardiovasc Res. 2003. PMID: 14613856
-
Muscarinic Stimulation Facilitates Sarcoplasmic Reticulum Ca Release by Modulating Ryanodine Receptor 2 Phosphorylation Through Protein Kinase G and Ca/Calmodulin-Dependent Protein Kinase II.Hypertension. 2016 Nov;68(5):1171-1178. doi: 10.1161/HYPERTENSIONAHA.116.07666. Epub 2016 Sep 19. Hypertension. 2016. PMID: 27647848 Free PMC article. Review.
Cited by
-
PI3K/Akt pathway mediates the positive inotropic effects of insulin in Langendorff-perfused rat hearts.Sci Rep. 2022 Jun 13;12(1):9793. doi: 10.1038/s41598-022-14092-2. Sci Rep. 2022. PMID: 35697740 Free PMC article.
-
Mitochondria-endoplasmic reticulum contacts in sepsis-induced myocardial dysfunction.Front Cell Dev Biol. 2022 Nov 24;10:1036225. doi: 10.3389/fcell.2022.1036225. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36506093 Free PMC article. Review.
-
Vericiguat suppresses ventricular tachyarrhythmias inducibility in a rabbit myocardial infarction model.PLoS One. 2024 Apr 16;19(4):e0301970. doi: 10.1371/journal.pone.0301970. eCollection 2024. PLoS One. 2024. PMID: 38626004 Free PMC article.
-
Muscarinic Receptors in Cardioprotection and Vascular Tone Regulation.Physiol Res. 2024 Apr 18;73(Suppl 1):S389-S400. doi: 10.33549/physiolres.935270. Epub 2024 Apr 18. Physiol Res. 2024. PMID: 38634650 Free PMC article. Review.
-
Multisite phosphorylation of the cardiac ryanodine receptor: a random or coordinated event?Pflugers Arch. 2020 Dec;472(12):1793-1807. doi: 10.1007/s00424-020-02473-3. Epub 2020 Oct 19. Pflugers Arch. 2020. PMID: 33078311 Review.
References
-
- Binkley P. F., Nunziata E., Haas G. J., Nelson S. D., and Cody R. J. (1991) Parasympathetic withdrawal is an integral component of autonomic imbalance in congestive heart failure: demonstration in human subjects and verification in a paced canine model of ventricular failure. J. Am. Coll. Cardiol. 18, 464–472 10.1016/0735-1097(91)90602-6 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

