Cardiac β-adrenergic receptor activation mediates distinct and cell type-dependent changes in the expression and distribution of connexin 43
- PMID: 32578931
- PMCID: PMC7412418
- DOI: 10.1111/jcmm.15469
Cardiac β-adrenergic receptor activation mediates distinct and cell type-dependent changes in the expression and distribution of connexin 43
Abstract
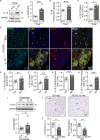
Activation of the sympatho-β-adrenergic receptors (β-ARs) system is a hallmark of heart failure, leading to fibrosis and arrhythmias. Connexin 43 (Cx43) is the most abundant gap junctional protein in the myocardium. Current knowledge is limited regarding Cx43 remodelling in diverse cell types in the diseased myocardium and the underlying mechanism. We studied cell type-dependent changes in Cx43 remodelling due to β-AR overactivation and molecular mechanisms involved. Mouse models of isoproterenol stimulation or transgenic cardiomyocyte overexpression of β2 -AR were used, which exhibited cardiac fibrosis and up-regulated total Cx43 abundance. In both models, whereas Cx43 expression in cardiomyocytes was reduced and more laterally distributed, fibroblasts exhibited elevated Cx43 expression and enhanced gap junction communication. Mechanistically, activation of β2 -AR in fibroblasts in vitro elevated Cx43 expression, which was abolished by the β2 -antagonist ICI-118551 or protein kinase A inhibitor H-89, but simulated by the adenylyl cyclase activator forskolin. Our in vitro and in vivo data showed that β-AR activation-induced production of IL-18 sequentially stimulated Cx43 expression in fibroblasts in a paracrine fashion. In summary, our findings demonstrate a pivotal role of β-AR in mediating distinct and cell type-dependent changes in the expression and distribution of Cx43, leading to pathological gap junction remodelling in the myocardium.
Keywords: IL-18; cardiac fibrosis; connexin 43; gap junction remodelling; β-AR activation.
© 2020 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
None declared.
Figures
Similar articles
-
Regulation of gap-junction protein connexin 43 by beta-adrenergic receptor stimulation in rat cardiomyocytes.Acta Pharmacol Sin. 2009 Jul;30(7):928-34. doi: 10.1038/aps.2009.92. Acta Pharmacol Sin. 2009. PMID: 19574999 Free PMC article.
-
Subchronic alpha- and beta-adrenergic regulation of cardiac gap junction protein expression.FASEB J. 2006 Feb;20(2):365-7. doi: 10.1096/fj.05-4871fje. Epub 2005 Dec 13. FASEB J. 2006. PMID: 16352648
-
Adrenergic control of cardiac gap junction function and expression.Naunyn Schmiedebergs Arch Pharmacol. 2011 Apr;383(4):331-46. doi: 10.1007/s00210-011-0603-4. Epub 2011 Feb 12. Naunyn Schmiedebergs Arch Pharmacol. 2011. PMID: 21318337 Review.
-
Opposing and synergistic effects of cyclic mechanical stretch and α- or β-adrenergic stimulation on the cardiac gap junction protein Cx43.Pharmacol Res. 2010 Dec;62(6):506-13. doi: 10.1016/j.phrs.2010.08.002. Epub 2010 Aug 10. Pharmacol Res. 2010. PMID: 20705136
-
[Remodeling of cardiac gap junctions and arrhythmias].Sheng Li Xue Bao. 2011 Dec 25;63(6):586-92. Sheng Li Xue Bao. 2011. PMID: 22193455 Review. Chinese.
Cited by
-
Hippo pathway activation mediates chemotherapy-induced anti-cancer effect and cardiomyopathy through causing mitochondrial damage and dysfunction.Theranostics. 2023 Jan 1;13(2):560-577. doi: 10.7150/thno.79227. eCollection 2023. Theranostics. 2023. PMID: 36632235 Free PMC article.
-
Adipocyte-released adipomes in Chagas cardiomyopathy: Impact on cardiac metabolic and immune regulation.iScience. 2024 Apr 5;27(5):109672. doi: 10.1016/j.isci.2024.109672. eCollection 2024 May 17. iScience. 2024. PMID: 38660407 Free PMC article.
-
Activation of Hippo signaling pathway mediates mitochondria dysfunction and dilated cardiomyopathy in mice.Theranostics. 2021 Aug 21;11(18):8993-9008. doi: 10.7150/thno.62302. eCollection 2021. Theranostics. 2021. PMID: 34522223 Free PMC article.
-
Atrial Fibrillation: Focus on Myocardial Connexins and Gap Junctions.Biology (Basel). 2022 Mar 23;11(4):489. doi: 10.3390/biology11040489. Biology (Basel). 2022. PMID: 35453689 Free PMC article. Review.
-
Sympatho-adrenergic mechanisms in heart failure: new insights into pathophysiology.Med Rev (2021). 2021 Oct 21;1(1):47-77. doi: 10.1515/mr-2021-0007. eCollection 2021 Oct. Med Rev (2021). 2021. PMID: 37724075 Free PMC article.
References
-
- Cohn JN, Levine TB, Olivari MT, et al. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med. 1984;311:819‐823. - PubMed
-
- Manolis AJ, Poulimenos LE, Kallistratos MS, Gavras I, Gavras H. Sympathetic overactivity in hypertension and cardiovascular disease. Curr Vasc Pharmacol. 2014;12:4‐15. - PubMed
-
- Nguyen MN, Kiriazis H, Ruggiero D, et al. Spontaneous ventricular tachyarrhythmias in beta2‐adrenoceptor transgenic mice in relation to cardiac interstitial fibrosis. Am J Physiol Heart Circ Physiol. 2015;309:H946‐H957. - PubMed
-
- Du XJ, Cox HS, Dart AM, Esler MD. Sympathetic activation triggers ventricular arrhythmias in rat heart with chronic infarction and failure. Cardiovasc Res. 1999;43:919‐929. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

