COP9 Signalosome Suppresses RIPK1-RIPK3-Mediated Cardiomyocyte Necroptosis in Mice
- PMID: 32578441
- PMCID: PMC7438278
- DOI: 10.1161/CIRCHEARTFAILURE.120.006996
COP9 Signalosome Suppresses RIPK1-RIPK3-Mediated Cardiomyocyte Necroptosis in Mice
Abstract
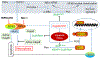
Background: Mechanisms governing the induction of heart failure by the impairment of autophagy and the ubiquitin-proteasome system and the molecular pathways to cardiomyocyte necrosis remain incompletely understood. COPS8 is an essential subunit of the COP9 (COnstitutive Photomorphogenesis 9) signalosome, a key regulator of ubiquitination. Mice with cardiomyocyte-restricted knockout of Cops8 (Cops8-cko) show autophagic and ubiquitin-proteasome system malfunction and massive cardiomyocyte necrosis followed by acute heart failure and premature death, providing an excellent animal model to address the mechanistic gaps specified above. This study was conducted to determine the nature and underlying mechanisms of the cardiomyocyte necrosis in Cops8-cko mice.
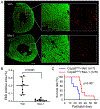
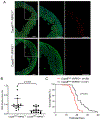
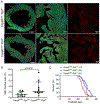
Methods and results: Compared with littermate control mice, myocardial protein levels of key factors in the necroptotic pathway (RIPK1 [receptor-interacting protein kinase 1], RIPK3, MLKL [mixed lineage kinase-like], the RIPK1-bound RIPK3), protein carbonyls, full-length Casp8 (caspase 8), and BCL2, as well as histochemical staining of superoxide anions were significantly higher but the cleaved Casp8 and the Casp8 activity were significantly lower in Cops8-cko mice. In vivo cardiomyocyte uptake of Evan's blue dye was used as an indicator of necrosis. Cops8-cko mice treated with a RIPK1 kinase inhibitor (Nec-1 [Necrostatin-1]) showed less Evans blue dye uptake (0.005% versus 0.20%; P<0.0001) and longer median lifespan (32.5 versus 27 days; P<0.01) than those treated with vehicle control. RIPK3 haploinsufficiency showed similar rescuing effects on Cops8-cko but Cyclophilin D deficiency did the opposite.
Conclusions: Cardiac Cops8/COP9 signalosome malfunction causes RIPK1-RIPK3 dependent, but mitochondrial permeability transition pore independent, cardiomyocyte necroptosis in mice and the COP9 signalosome plays an indispensable role in suppressing cardiomyocyte necroptosis.
Trial registration: ClinicalTrials.gov NCT04248894.
Keywords: caspase 8; mice; myocytes, cardiac; necrosis; ubiquitination.
Figures



Similar articles
-
Cullin Deneddylation Suppresses the Necroptotic Pathway in Cardiomyocytes.Front Physiol. 2021 Jun 28;12:690423. doi: 10.3389/fphys.2021.690423. eCollection 2021. Front Physiol. 2021. PMID: 34262479 Free PMC article.
-
The neurotoxicant PCB-95 by increasing the neuronal transcriptional repressor REST down-regulates caspase-8 and increases Ripk1, Ripk3 and MLKL expression determining necroptotic neuronal death.Biochem Pharmacol. 2017 Oct 15;142:229-241. doi: 10.1016/j.bcp.2017.06.135. Epub 2017 Jul 1. Biochem Pharmacol. 2017. PMID: 28676433
-
Caspase-8 Blocks Receptor-Interacting Protein Kinase-1 Kinase-Independent Necroptosis during Embryogenesis.Immunohorizons. 2022 Jul 20;6(7):465-475. doi: 10.4049/immunohorizons.2200021. Immunohorizons. 2022. PMID: 35858757
-
The Inflammatory Signal Adaptor RIPK3: Functions Beyond Necroptosis.Int Rev Cell Mol Biol. 2017;328:253-275. doi: 10.1016/bs.ircmb.2016.08.007. Epub 2016 Sep 22. Int Rev Cell Mol Biol. 2017. PMID: 28069136 Free PMC article. Review.
-
Necroptosis in development and diseases.Genes Dev. 2018 Mar 1;32(5-6):327-340. doi: 10.1101/gad.312561.118. Genes Dev. 2018. PMID: 29593066 Free PMC article. Review.
Cited by
-
NETosis as a Pathogenic Factor for Heart Failure.Oxid Med Cell Longev. 2021 Feb 23;2021:6687096. doi: 10.1155/2021/6687096. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 33680285 Free PMC article. Review.
-
The necessity of NEDD8/Rub1 for vitality and its association with mitochondria-derived oxidative stress.Redox Biol. 2020 Oct;37:101765. doi: 10.1016/j.redox.2020.101765. Epub 2020 Oct 20. Redox Biol. 2020. PMID: 33099217 Free PMC article. Review.
-
Protein neddylation and its role in health and diseases.Signal Transduct Target Ther. 2024 Apr 5;9(1):85. doi: 10.1038/s41392-024-01800-9. Signal Transduct Target Ther. 2024. PMID: 38575611 Free PMC article. Review.
-
The Role of Cullin-RING Ligases in Striated Muscle Development, Function, and Disease.Int J Mol Sci. 2020 Oct 26;21(21):7936. doi: 10.3390/ijms21217936. Int J Mol Sci. 2020. PMID: 33114658 Free PMC article. Review.
-
Cell death in head and neck cancer pathogenesis and treatment.Cell Death Dis. 2021 Feb 18;12(2):192. doi: 10.1038/s41419-021-03474-5. Cell Death Dis. 2021. PMID: 33602906 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

