Ocular co-Delivery of Timolol and Brimonidine from a Self-Assembling Peptide Hydrogel for the Treatment of Glaucoma: In Vitro and Ex Vivo Evaluation
- PMID: 32575910
- PMCID: PMC7344471
- DOI: 10.3390/ph13060126
Ocular co-Delivery of Timolol and Brimonidine from a Self-Assembling Peptide Hydrogel for the Treatment of Glaucoma: In Vitro and Ex Vivo Evaluation
Abstract
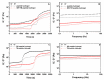
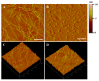
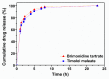
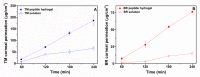
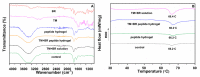
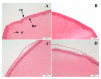
Effective pharmacotherapy during glaucoma treatment depends on interventions that reduce intraocular pressure (IOP) and retain the IOP lowering effect for sufficient time so as to reduce dosing frequency and enhance patient adherence. Combination anti-glaucoma therapy and dosage forms that increase precorneal residence time could therefore constitute a promising therapeutic intervention. The in-situ gel forming self-assembling peptide ac-(RADA)4-CONH2 was evaluated as carrier for the ocular co-delivery of timolol maleate (TM) and brimonidine tartrate (BR). The hydrogel's microstructure and mechanical properties were assessed with atomic force microscopy and rheology, respectively. Drug diffusion from the hydrogel was evaluated in vitro in simulated tear fluid and ex vivo across porcine corneas and its effect on the treated corneas was assessed through physicochemical characterization and histological analysis. Results indicated that TM and BR co-delivery affected hydrogel's microstructure resulting in shorter nanofibers and a less rigid hydrogel matrix. Rapid and complete release of both drugs was achieved within 8 h, while a 2.8-fold and 5.4-fold higher corneal permeability was achieved for TM and BR, respectively. No significant alterations were induced in the structural integrity of the corneas treated with the hydrogel formulation, suggesting that self-assembling peptide hydrogels might serve as promising systems for combination anti-glaucoma therapy.
Keywords: ex vivo ocular permeability; glaucoma; self-assembling peptide hydrogel; timolol maleate/brimonidine tartrate combination.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Cationic liposomes as promising vehicles for timolol/brimonidine combination ocular delivery in glaucoma: formulation development and in vitro/in vivo evaluation.Drug Deliv Transl Res. 2023 Apr;13(4):1035-1047. doi: 10.1007/s13346-022-01266-8. Epub 2022 Dec 7. Drug Deliv Transl Res. 2023. PMID: 36477776
-
Self-Assembling Peptide Nanofiber Hydrogels for Controlled Ocular Delivery of Timolol Maleate.ACS Biomater Sci Eng. 2017 Dec 11;3(12):3386-3394. doi: 10.1021/acsbiomaterials.7b00706. Epub 2017 Nov 16. ACS Biomater Sci Eng. 2017. PMID: 33445378
-
Formulation and evaluation of stimuli-sensitive hydrogels of timolol maleate and brimonidine tartrate for the treatment of glaucoma.Int J Pharm Investig. 2014 Jul;4(3):112-8. doi: 10.4103/2230-973X.138340. Int J Pharm Investig. 2014. PMID: 25126524 Free PMC article.
-
Topical brimonidine 0.2%/timolol 0.5% ophthalmic solution: in glaucoma and ocular hypertension.Drugs Aging. 2006;23(9):753-61. doi: 10.2165/00002512-200623090-00005. Drugs Aging. 2006. PMID: 17020399 Review.
-
Neuroprotection for treatment of glaucoma in adults.Cochrane Database Syst Rev. 2013 Feb 28;2(2):CD006539. doi: 10.1002/14651858.CD006539.pub3. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2017 Jan 25;1:CD006539. doi: 10.1002/14651858.CD006539.pub4. PMID: 23450569 Free PMC article. Updated. Review.
Cited by
-
Overview of Recent Advances in Nano-Based Ocular Drug Delivery.Int J Mol Sci. 2023 Oct 19;24(20):15352. doi: 10.3390/ijms242015352. Int J Mol Sci. 2023. PMID: 37895032 Free PMC article. Review.
-
Simple Complexity: Incorporating Bioinspired Delivery Machinery within Self-Assembled Peptide Biogels.Gels. 2023 Mar 6;9(3):199. doi: 10.3390/gels9030199. Gels. 2023. PMID: 36975648 Free PMC article. Review.
-
Ocular Delivery of Quercetin Using Microemulsion System: Design, Characterization, and Ex-vivo Transcorneal Permeation.Iran J Pharm Res. 2022 Aug 20;21(1):e127486. doi: 10.5812/ijpr-127486. eCollection 2022 Dec. Iran J Pharm Res. 2022. PMID: 36945341 Free PMC article.
-
Nanofibers in Ocular Drug Targeting and Tissue Engineering: Their Importance, Advantages, Advances, and Future Perspectives.Pharmaceutics. 2023 Mar 25;15(4):1062. doi: 10.3390/pharmaceutics15041062. Pharmaceutics. 2023. PMID: 37111550 Free PMC article. Review.
-
Cationic liposomes as promising vehicles for timolol/brimonidine combination ocular delivery in glaucoma: formulation development and in vitro/in vivo evaluation.Drug Deliv Transl Res. 2023 Apr;13(4):1035-1047. doi: 10.1007/s13346-022-01266-8. Epub 2022 Dec 7. Drug Deliv Transl Res. 2023. PMID: 36477776
References
LinkOut - more resources
Full Text Sources

