Integrin-Targeting Dye-Doped PEG-Shell/Silica-Core Nanoparticles Mimicking the Proapoptotic Smac/DIABLO Protein
- PMID: 32575872
- PMCID: PMC7353088
- DOI: 10.3390/nano10061211
Integrin-Targeting Dye-Doped PEG-Shell/Silica-Core Nanoparticles Mimicking the Proapoptotic Smac/DIABLO Protein
Abstract
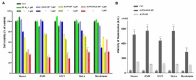
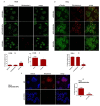
Cancer cells demonstrate elevated expression levels of the inhibitor of apoptosis proteins (IAPs), contributing to tumor cell survival, disease progression, chemo-resistance, and poor prognosis. Smac/DIABLO is a mitochondrial protein that promotes apoptosis by neutralizing members of the IAP family. Herein, we describe the preparation and in vitro validation of a synthetic mimic of Smac/DIABLO, based on fluorescent polyethylene glycol (PEG)-coated silica-core nanoparticles (NPs) carrying a Smac/DIABLO-derived pro-apoptotic peptide and a tumor-homing integrin peptide ligand. At low μM concentration, the NPs showed significant toxicity towards A549, U373, and HeLa cancer cells and modest toxicity towards other integrin-expressing cells, correlated with integrin-mediated cell uptake and consequent highly increased levels of apoptotic activity, without perturbing cells not expressing the α5 integrin subunit.
Keywords: AVPI; IAP; RGD; Smac/DIABLO; cancer; cellular uptake; confocal microscopy; drug delivery; silica nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Is there something else besides the proapoptotic AVPI-segment in the Smac/DIABLO protein?Bol Med Hosp Infant Mex. 2016 Nov-Dec;73(6):365-371. doi: 10.1016/j.bmhimx.2016.10.004. Epub 2016 Nov 30. Bol Med Hosp Infant Mex. 2016. PMID: 29421280 Review.
-
X-linked inhibitor of apoptosis (XIAP) blocks Apo2 ligand/tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis of prostate cancer cells in the presence of mitochondrial activation: sensitization by overexpression of second mitochondria-derived activator of caspase/direct IAP-binding protein with low pl (Smac/DIABLO).Mol Cancer Ther. 2002 Oct;1(12):1051-8. Mol Cancer Ther. 2002. PMID: 12481428
-
Neutralization of Smac/Diablo by inhibitors of apoptosis (IAPs). A caspase-independent mechanism for apoptotic inhibition.J Biol Chem. 2004 Dec 3;279(49):51082-90. doi: 10.1074/jbc.M408655200. Epub 2004 Sep 15. J Biol Chem. 2004. PMID: 15371416
-
Rapid induction of IAP family proteins and Smac/DIABLO expression after proapoptotic stimulation with doxorubicin in RPMI 8226 multiple myeloma cells.Exp Mol Pathol. 2007 Dec;83(3):405-12. doi: 10.1016/j.yexmp.2007.04.001. Epub 2007 Apr 18. Exp Mol Pathol. 2007. PMID: 17521628
-
Smac/DIABLO and colon cancer.Anticancer Agents Med Chem. 2007 Jul;7(4):467-73. doi: 10.2174/187152007781058631. Anticancer Agents Med Chem. 2007. PMID: 17630921 Review.
Cited by
-
Dual-targeting peptides@PMO, a mimetic to the pro-apoptotic protein Smac/DIABLO for selective activation of apoptosis in cancer cells.Front Pharmacol. 2023 Aug 29;14:1237478. doi: 10.3389/fphar.2023.1237478. eCollection 2023. Front Pharmacol. 2023. PMID: 37711175 Free PMC article.
-
Integrin-Targeting Peptides for the Design of Functional Cell-Responsive Biomaterials.Biomedicines. 2020 Aug 25;8(9):307. doi: 10.3390/biomedicines8090307. Biomedicines. 2020. PMID: 32854363 Free PMC article. Review.
-
Design of α/β-Hybrid Peptide Ligands of α4β1 Integrin Equipped with a Linkable Side Chain for Chemoselective Biofunctionalization of Microstructured Materials.Biomedicines. 2021 Nov 21;9(11):1737. doi: 10.3390/biomedicines9111737. Biomedicines. 2021. PMID: 34829965 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

