Three Novel COVID-19 Pneumonia Cases Successfully Treated With Lopinavir/Ritonavir
- PMID: 32574332
- PMCID: PMC7248319
- DOI: 10.3389/fmed.2020.00241
Three Novel COVID-19 Pneumonia Cases Successfully Treated With Lopinavir/Ritonavir
Abstract
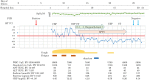
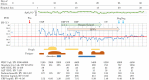
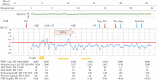
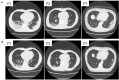
Following the first case of Coronavirus Disease 2019 (COVID-19), caused by Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-Cov-2), in Wuhan, China, in December 2019, it has spread worldwide. An outbreak in Japan occurred on a cruise ship, and this was followed by community-acquired COVID-19. Herein, we report three cases of COVID-19 that presented pneumonia following admission to Kitasato University Hospital. Patients were admitted based on the positive result of real-time reverse transcriptase-polymerase chain reaction (RT-PCR) tests for COVID-19 nucleic acid. All patients were diagnosed as suffering from non-severe COVID-19 pneumonia and were successfully treated with Lopinavir/Ritonavir (LPV/r). LPV/r could be an option for treating non-severe COVID-19 pneumonia in general and even in elderly patients.
Keywords: COVID-19; Lopinavir; Ritonavir; cruise ship; elderly patient; hyponatremia.
Copyright © 2020 Wada, Shimode, Hoshiyama, Takayama and Yamaoka.
Figures
Similar articles
-
Four Cases of Coronavirus Disease 2019 Transferred from a Cruise Ship.Intern Med. 2021 Feb 1;60(3):479-485. doi: 10.2169/internalmedicine.4939-20. Epub 2020 Dec 22. Intern Med. 2021. PMID: 33361672 Free PMC article.
-
COVID-19 can suddenly become severe: a case series from Tokyo, Japan.Glob Health Med. 2020 Jun 30;2(3):174-177. doi: 10.35772/ghm.2020.01054. Glob Health Med. 2020. PMID: 33330803 Free PMC article.
-
Successful recovery from critical COVID-19 pneumonia with extracorporeal membrane oxygenation: A case report.Respir Med Case Rep. 2020 May 31;30:101113. doi: 10.1016/j.rmcr.2020.101113. eCollection 2020. Respir Med Case Rep. 2020. PMID: 32523870 Free PMC article.
-
Influence of COVID-19 on Cerebrovascular Disease and its Possible Mechanism.Neuropsychiatr Dis Treat. 2020 May 28;16:1359-1367. doi: 10.2147/NDT.S251173. eCollection 2020. Neuropsychiatr Dis Treat. 2020. PMID: 32547039 Free PMC article. Review.
-
Protease Inhibitor Use in COVID-19.SN Compr Clin Med. 2020;2(9):1436-1443. doi: 10.1007/s42399-020-00448-0. Epub 2020 Aug 14. SN Compr Clin Med. 2020. PMID: 32838187 Free PMC article. Review.
Cited by
-
An Update on Antiviral Therapy Against SARS-CoV-2: How Far Have We Come?Front Pharmacol. 2021 Mar 8;12:632677. doi: 10.3389/fphar.2021.632677. eCollection 2021. Front Pharmacol. 2021. PMID: 33762954 Free PMC article. Review.
-
Risk factors for prolonged virus shedding of respiratory tract and fecal in adults with severe acute respiratory syndrome coronavirus-2 infection.J Clin Lab Anal. 2021 Sep;35(9):e23923. doi: 10.1002/jcla.23923. Epub 2021 Aug 13. J Clin Lab Anal. 2021. PMID: 34390043 Free PMC article.
-
Repurposing Approved Drugs for Guiding COVID-19 Prophylaxis: A Systematic Review.Front Pharmacol. 2020 Dec 14;11:590598. doi: 10.3389/fphar.2020.590598. eCollection 2020. Front Pharmacol. 2020. PMID: 33390967 Free PMC article. Review.
-
Clinical Management of COVID-19: A Review of Pharmacological Treatment Options.Pharmaceuticals (Basel). 2021 May 28;14(6):520. doi: 10.3390/ph14060520. Pharmaceuticals (Basel). 2021. PMID: 34071185 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

