Metformin induces caspase-dependent and caspase-independent apoptosis in human bladder cancer T24 cells
- PMID: 32568826
- PMCID: PMC7365670
- DOI: 10.1097/CAD.0000000000000966
Metformin induces caspase-dependent and caspase-independent apoptosis in human bladder cancer T24 cells
Abstract
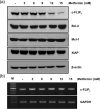
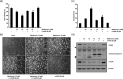
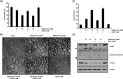
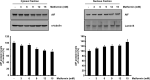
Bladder cancer (BC) is the sixth most common cancer in men. Moreover, chemotherapy for BC leads to various side effects. Metformin is known to induce apoptosis in vitro in many types of cancer. Furthermore, it has feasibility as a drug repositioning used for the treatment of cancer. The molecular mechanism of metformin mediating apoptosis in BC is still unclear. In this study, we showed that metformin stimulated the caspase-dependent apoptotic signaling pathway in T24 cells, a human BC cell line. Moreover, the induced apoptosis was partially inhibited by a general caspase inhibitor, z-VAD-fmk, which suggested that metformin-induced apoptosis in T24 cells is partially caspase-independent. Notably, we observed the nuclear translocation of apoptosis-inducing factors (AIFs) in metformin-promoted apoptosis, which is a typical characteristic of the caspase-independent apoptotic pathway. In addition, we found that metformin-mediated apoptosis occurred via degradation of the cellular FADD-like interleukin-1β-converting enzyme inhibitory protein (c-FLIP) by facilitating ubiquitin/proteasome-mediated c-FLIPL degradation. Furthermore, treatment with the reactive oxygen species scavenger N-acetylcysteine, failed to suppress metformin-induced apoptosis and c-FLIPL protein degradation in metformin-treated T24 cells. In conclusion, these results indicate that metformin-induced apoptosis was mediated through AIF-promoted caspase-independent pathways as well as caspase-dependent pathways in T24 cells. As such, metformin could be used as a possible apoptotic agent for the treatment of BC.
Conflict of interest statement
There are no conflictss of interest.
Figures

Similar articles
-
Metformin sensitizes human bladder cancer cells to TRAIL-induced apoptosis through mTOR/S6K1-mediated downregulation of c-FLIP.Anticancer Drugs. 2014 Sep;25(8):887-97. doi: 10.1097/CAD.0000000000000116. Anticancer Drugs. 2014. PMID: 24714080
-
The antidiabetic drug ciglitazone induces high grade bladder cancer cells apoptosis through the up-regulation of TRAIL.PLoS One. 2011;6(12):e28354. doi: 10.1371/journal.pone.0028354. Epub 2011 Dec 12. PLoS One. 2011. PMID: 22174792 Free PMC article.
-
ITCH-dependent proteasomal degradation of c-FLIP induced by the anti-HER3 antibody 9F7-F11 promotes DR5/caspase 8-mediated apoptosis of tumor cells.Cell Commun Signal. 2019 Aug 23;17(1):106. doi: 10.1186/s12964-019-0413-8. Cell Commun Signal. 2019. PMID: 31443721 Free PMC article.
-
A novel synthetic 2-(3-methoxyphenyl)-6,7-methylenedioxoquinolin-4-one arrests the G2/M phase arrest via Cdc25c and induces apoptosis through caspase- and mitochondria-dependent pathways in TSGH8301 human bladder cancer cells.Int J Oncol. 2012 Mar;40(3):731-8. doi: 10.3892/ijo.2011.1241. Epub 2011 Oct 21. Int J Oncol. 2012. PMID: 22021033
-
c-FLIP, a master anti-apoptotic regulator.Exp Oncol. 2012 Oct;34(3):176-84. Exp Oncol. 2012. PMID: 23070002 Free PMC article. Review.
Cited by
-
Characterization of the Lipid Metabolism in Bladder Cancer to Guide Clinical Therapy.J Oncol. 2022 Sep 12;2022:7679652. doi: 10.1155/2022/7679652. eCollection 2022. J Oncol. 2022. PMID: 36131793 Free PMC article.
-
The anti-inflammatory effect of metformin: The molecular targets.Genes Cells. 2024 Mar;29(3):183-191. doi: 10.1111/gtc.13098. Epub 2024 Feb 4. Genes Cells. 2024. PMID: 38311861 Free PMC article. Review.
-
A Mini Review on Molecules Inducing Caspase-Independent Cell Death: A New Route to Cancer Therapy.Molecules. 2022 Sep 28;27(19):6401. doi: 10.3390/molecules27196401. Molecules. 2022. PMID: 36234938 Free PMC article. Review.
References
-
- Janković S, Radosavljević V. Risk factors for bladder cancer. Tumori. 2007; 93:4–12 - PubMed
-
- Grayson M. Bladder cancer. Nature. 2017; 551:S33. - PubMed
-
- Bladder cancer: diagnosis and management of bladder cancer: (c) NICE (2015) Bladder cancer: diagnosis and management of bladder cancer. BJU Int. 2017; 120:755–765 - PubMed
-
- Gadducci A, Biglia N, Tana R, Cosio S, Gallo M. Metformin use and gynecological cancers: a novel treatment option emerging from drug repositioning. Crit Rev Oncol Hematol. 2016; 105:73–83 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

