Polyphenols as Potential Metal Chelation Compounds Against Alzheimer's Disease
- PMID: 32568200
- PMCID: PMC7809605
- DOI: 10.3233/JAD-200185
Polyphenols as Potential Metal Chelation Compounds Against Alzheimer's Disease
Abstract
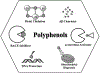
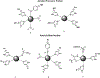
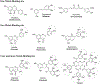
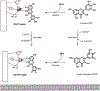
Alzheimer's disease (AD) is the most common neurodegenerative disease affecting more than 50 million people worldwide. The pathology of this multifactorial disease is primarily characterized by the formation of amyloid-β (Aβ) aggregates; however, other etiological factors including metal dyshomeostasis, specifically copper (Cu), zinc (Zn), and iron (Fe), play critical role in disease progression. Because these transition metal ions are important for cellular function, their imbalance can cause oxidative stress that leads to cellular death and eventual cognitive decay. Importantly, these transition metal ions can interact with the amyloid-β protein precursor (AβPP) and Aβ42 peptide, affecting Aβ aggregation and increasing its neurotoxicity. Considering how metal dyshomeostasis may substantially contribute to AD, this review discusses polyphenols and the underlying chemical principles that may enable them to act as natural chelators. Furthermore, polyphenols have various therapeutic effects, including antioxidant activity, metal chelation, mitochondrial function, and anti-amyloidogenic activity. These combined therapeutic effects of polyphenols make them strong candidates for a moderate chelation-based therapy for AD.
Keywords: Alzheimer’s disease; amyloid-β; copper; iron; metal chelation therapy; metalloproteins; polyphenols; zinc.
Figures
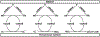

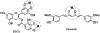
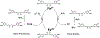

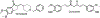
Similar articles
-
The impact of chelating compounds on Cu2+, Fe2+/3+, and Zn2+ ions in Alzheimer's disease treatment.J Inorg Biochem. 2024 Aug;257:112601. doi: 10.1016/j.jinorgbio.2024.112601. Epub 2024 May 9. J Inorg Biochem. 2024. PMID: 38744143 Review.
-
Management of oxidative stress and other pathologies in Alzheimer's disease.Arch Toxicol. 2019 Sep;93(9):2491-2513. doi: 10.1007/s00204-019-02538-y. Epub 2019 Aug 22. Arch Toxicol. 2019. PMID: 31440798 Review.
-
Metallobiology and therapeutic chelation of biometals (copper, zinc and iron) in Alzheimer's disease: Limitations, and current and future perspectives.J Trace Elem Med Biol. 2021 Sep;67:126779. doi: 10.1016/j.jtemb.2021.126779. Epub 2021 May 15. J Trace Elem Med Biol. 2021. PMID: 34034029 Review.
-
Copper-mediated β-amyloid toxicity and its chelation therapy in Alzheimer's disease.Metallomics. 2022 Jun 13;14(6):mfac018. doi: 10.1093/mtomcs/mfac018. Metallomics. 2022. PMID: 35333348 Review.
-
Rebalancing metal dyshomeostasis for Alzheimer's disease therapy.J Biol Inorg Chem. 2019 Dec;24(8):1159-1170. doi: 10.1007/s00775-019-01712-y. Epub 2019 Sep 5. J Biol Inorg Chem. 2019. PMID: 31486954 Review.
Cited by
-
Do Rutin and Quercetin Retain Their Structure and Radical Scavenging Activity after Exposure to Radiation?Molecules. 2023 Mar 17;28(6):2713. doi: 10.3390/molecules28062713. Molecules. 2023. PMID: 36985686 Free PMC article.
-
Characterization and Valorization of the Agricultural Waste Obtained from Lavandula Steam Distillation for Its Reuse in the Food and Pharmaceutical Fields.Molecules. 2022 Feb 28;27(5):1613. doi: 10.3390/molecules27051613. Molecules. 2022. PMID: 35268713 Free PMC article.
-
(-)-Epigallocatechin-3-Gallate Diminishes Intra-and Extracellular Amyloid-Induced Cytotoxic Effects on Cholinergic-like Neurons from Familial Alzheimer's Disease PSEN1 E280A.Biomolecules. 2021 Dec 8;11(12):1845. doi: 10.3390/biom11121845. Biomolecules. 2021. PMID: 34944489 Free PMC article.
-
The Chelating Ability of Plant Polyphenols Can Affect Iron Homeostasis and Gut Microbiota.Antioxidants (Basel). 2023 Mar 3;12(3):630. doi: 10.3390/antiox12030630. Antioxidants (Basel). 2023. PMID: 36978878 Free PMC article. Review.
-
Spectroscopic, Electrochemical, and Biological Assays of Copper-Binding Molecules for Screening of Different Drugs and Plant Extracts against Neurodegenerative Disorders.ACS Omega. 2022 May 4;7(19):16260-16269. doi: 10.1021/acsomega.1c03378. eCollection 2022 May 17. ACS Omega. 2022. PMID: 35601340 Free PMC article.
References
-
- Lakey-Beitia J, Berrocal R, Rao KS, Durant AA (2015) Polyphenols as therapeutic molecules in Alzheimer’s disease through modulating amyloid pathways. Mol Neurobiol 51, 466–479. - PubMed
-
- Wang H, Dharmalingam P, Vasquez V, Mitra J, Boldogh I, Rao KS, Kent TA, Mitra S, Hegde ML (2017) Chronic oxidative damage together with genome repair deficiency in the neurons is a double whammy for neurodegeneration: Is damage response signaling a potential therapeutic target? Mech Ageing Dev 161, 163–176. - PMC - PubMed
-
- Shaw BF, Valentine JS (2007) How do ALS-associated mutations in superoxide dismutase 1 promote aggregation of the protein? Trends Biochem Sci 32, 78–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

