Long-distance modulation of bystander tumor cells by CD8+ T cell-secreted IFNγ
- PMID: 32566933
- PMCID: PMC7305033
- DOI: 10.1038/s43018-020-0036-4
Long-distance modulation of bystander tumor cells by CD8+ T cell-secreted IFNγ
Erratum in
-
Publisher Correction: Long-distance modulation of bystander tumor cells by CD8+ T-cell-secreted IFN-γ.Nat Cancer. 2020 Jul;1(7):749. doi: 10.1038/s43018-020-0092-9. Nat Cancer. 2020. PMID: 35122043 No abstract available.
Abstract
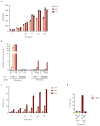
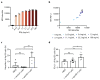
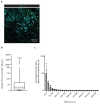
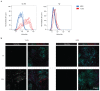
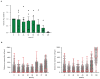
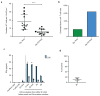
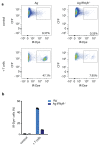
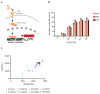
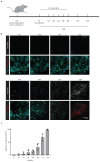
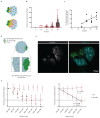
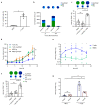
T cell-secreted IFNγ can exert pleiotropic effects on tumor cells that include induction of immune checkpoints and antigen presentation machinery components, and inhibition of cell growth. Despite its role as key effector molecule, little is known about the spatiotemporal spreading of IFNγ secreted by activated CD8+ T cells within the tumor environment. Using multiday intravital imaging, we demonstrate that T cell recognition of a minor fraction of tumor cells leads to sensing of IFNγ by a large part of the tumor mass. Furthermore, imaging of tumors in which antigen-positive and -negative tumor cells are separated in space reveals spreading of the IFNγ response, reaching distances of >800 µm. Notably, long-range sensing of IFNγ can modify tumor behavior, as both shown by induction of PD-L1 expression and inhibition of tumor growth. Collectively, these data reveal how, through IFNγ, CD8+ T cells modulate the behavior of remote tumor cells, including antigen-loss variants.
Keywords: CD8+ T cells; IFNγ receptor signaling reporter; IFNγ spreading; intravital imaging; tumor immunology.
Conflict of interest statement
Competing interests The authors declare no competing financial interests.
Figures


Comment in
-
Carpet-bombing tumors with IFN-γ.Nat Cancer. 2020 Mar;1(3):270-272. doi: 10.1038/s43018-020-0042-6. Nat Cancer. 2020. PMID: 35122031 No abstract available.
Similar articles
-
Modulation of the tumor micro-environment by CD8+ T cell-derived cytokines.Curr Opin Immunol. 2021 Apr;69:65-71. doi: 10.1016/j.coi.2021.03.016. Epub 2021 Apr 13. Curr Opin Immunol. 2021. PMID: 33862306 Free PMC article. Review.
-
Irradiation Suppresses IFNγ-Mediated PD-L1 and MCL1 Expression in EGFR-Positive Lung Cancer to Augment CD8+ T Cells Cytotoxicity.Cells. 2021 Sep 23;10(10):2515. doi: 10.3390/cells10102515. Cells. 2021. PMID: 34685495 Free PMC article.
-
IFNγ producing CD8+ T cells modified to resist major immune checkpoints induce regression of MHC class I-deficient melanomas.Oncoimmunology. 2015 Mar 6;4(2):e974959. doi: 10.4161/2162402X.2014.974959. eCollection 2015 Feb. Oncoimmunology. 2015. PMID: 25949872 Free PMC article.
-
Distinct spatiotemporal dynamics of CD8+ T cell-derived cytokines in the tumor microenvironment.Cancer Cell. 2024 Jan 8;42(1):157-167.e9. doi: 10.1016/j.ccell.2023.12.010. Cancer Cell. 2024. PMID: 38194914 Free PMC article.
-
Naïve CD8 T cell IFNγ responses to a vacuolar antigen are regulated by an inflammasome-independent NLRP3 pathway and Toxoplasma gondii ROP5.PLoS Pathog. 2020 Aug 27;16(8):e1008327. doi: 10.1371/journal.ppat.1008327. eCollection 2020 Aug. PLoS Pathog. 2020. PMID: 32853276 Free PMC article.
Cited by
-
Transcriptional regulation of DLGAP5 by AR suppresses p53 signaling and inhibits CD8+T cell infiltration in triple-negative breast cancer.Transl Oncol. 2024 Nov;49:102081. doi: 10.1016/j.tranon.2024.102081. Epub 2024 Aug 24. Transl Oncol. 2024. PMID: 39182361 Free PMC article.
-
Modulation of the tumor micro-environment by CD8+ T cell-derived cytokines.Curr Opin Immunol. 2021 Apr;69:65-71. doi: 10.1016/j.coi.2021.03.016. Epub 2021 Apr 13. Curr Opin Immunol. 2021. PMID: 33862306 Free PMC article. Review.
-
Interferon-induced factor 16 is essential in metastatic melanoma to maintain STING levels and the immune responses upon IFN-γ response pathway activation.J Immunother Cancer. 2024 Oct 18;12(10):e009590. doi: 10.1136/jitc-2024-009590. J Immunother Cancer. 2024. PMID: 39424359 Free PMC article.
-
In vivo imaging of inflammatory response in cancer research.Inflamm Regen. 2023 Feb 7;43(1):10. doi: 10.1186/s41232-023-00261-x. Inflamm Regen. 2023. PMID: 36750856 Free PMC article. Review.
-
Rational combinations of targeted cancer therapies: background, advances and challenges.Nat Rev Drug Discov. 2023 Mar;22(3):213-234. doi: 10.1038/s41573-022-00615-z. Epub 2022 Dec 12. Nat Rev Drug Discov. 2023. PMID: 36509911 Review.
References
-
- Galon J, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

