PSI-MOUSE: Predicting Mouse Pseudouridine Sites From Sequence and Genome-Derived Features
- PMID: 32565674
- PMCID: PMC7285933
- DOI: 10.1177/1176934320925752
PSI-MOUSE: Predicting Mouse Pseudouridine Sites From Sequence and Genome-Derived Features
Abstract
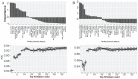
Pseudouridine (Ψ) is the first discovered and the most prevalent posttranscriptional modification, which has been widely studied during the past decades. Pseudouridine was observed in almost all kinds of RNAs and shown to have important biological functions. Currently, the time-consuming and high-cost procedures of experimental approaches limit its uses in real-life Ψ site detection. Alternatively, by taking advantage of the explosive growth of Ψ sequencing data, the computational methods may provide a more cost-effective avenue. To date, the existing mouse Ψ site predictors were all developed based on sequence-derived features, and their performance can be further improved by adding the domain knowledge derived feature. Therefore, it is highly desirable to propose a genomic feature-based computational method to increase the accuracy and efficiency of the identification of Ψ RNA modification in the mouse transcriptome. In our study, a predictive framework PSI-MOUSE was built. Besides the conventional sequence-based features, PSI-MOUSE first introduced 38 additional genomic features derived from the mouse genome, which achieved a satisfactory improvement in the prediction performance, compared with other existing models. Moreover, PSI-MOUSE also features in automatically annotating the putative Ψ sites with diverse types of posttranscriptional regulations (RNA-binding protein [RBP]-binding regions, miRNA-RNA interactions, and splicing sites), which can serve as a useful research tool for the study of Ψ RNA modification in the mouse genome. Finally, 3282 experimentally validated mouse Ψ sites were also collected in a database with customized query functions. For the convenience of academic users, a website was built to provide a user-friendly interface for the query and analysis on the database. The website is freely accessible at www.xjtlu.edu.cn/biologicalsciences/psimouse and http://psimouse.rnamd.com. We introduced the genome-derived features to mouse for the first time, and we achieved a good performance in mouse Ψ site prediction. Compared with the existing state-of-art methods, our newly developed approach PSI-MOUSE obtained a substantial improvement in prediction accuracy, marking the reliable contributions of genomic features for the prediction of RNA modifications in a species other than human.
Keywords: Pseudouridine sites; genomic feature; web-server.
© The Author(s) 2020.
Conflict of interest statement
Declaration of Conflicting Interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures

Similar articles
-
PIANO: A Web Server for Pseudouridine-Site (Ψ) Identification and Functional Annotation.Front Genet. 2020 Mar 12;11:88. doi: 10.3389/fgene.2020.00088. eCollection 2020. Front Genet. 2020. PMID: 32226440 Free PMC article.
-
Pseudouridine Identification and Functional Annotation with PIANO.Methods Mol Biol. 2023;2624:153-162. doi: 10.1007/978-1-0716-2962-8_11. Methods Mol Biol. 2023. PMID: 36723815
-
PseUI: Pseudouridine sites identification based on RNA sequence information.BMC Bioinformatics. 2018 Aug 29;19(1):306. doi: 10.1186/s12859-018-2321-0. BMC Bioinformatics. 2018. PMID: 30157750 Free PMC article.
-
Comprehensive review and assessment of computational methods for predicting RNA post-transcriptional modification sites from RNA sequences.Brief Bioinform. 2020 Sep 25;21(5):1676-1696. doi: 10.1093/bib/bbz112. Brief Bioinform. 2020. PMID: 31714956 Review.
-
BID-seq for transcriptome-wide quantitative sequencing of mRNA pseudouridine at base resolution.Nat Protoc. 2024 Feb;19(2):517-538. doi: 10.1038/s41596-023-00917-5. Epub 2023 Nov 15. Nat Protoc. 2024. PMID: 37968414 Review.
Cited by
-
Machine learning applications in RNA modification sites prediction.Comput Struct Biotechnol J. 2021 Sep 29;19:5510-5524. doi: 10.1016/j.csbj.2021.09.025. eCollection 2021. Comput Struct Biotechnol J. 2021. PMID: 34712397 Free PMC article. Review.
-
Porpoise: a new approach for accurate prediction of RNA pseudouridine sites.Brief Bioinform. 2021 Nov 5;22(6):bbab245. doi: 10.1093/bib/bbab245. Brief Bioinform. 2021. PMID: 34226915 Free PMC article.
-
Dynamic regulation and key roles of ribonucleic acid methylation.Front Cell Neurosci. 2022 Dec 19;16:1058083. doi: 10.3389/fncel.2022.1058083. eCollection 2022. Front Cell Neurosci. 2022. PMID: 36601431 Free PMC article. Review.
-
RMDisease: a database of genetic variants that affect RNA modifications, with implications for epitranscriptome pathogenesis.Nucleic Acids Res. 2021 Jan 8;49(D1):D1396-D1404. doi: 10.1093/nar/gkaa790. Nucleic Acids Res. 2021. PMID: 33010174 Free PMC article.
-
Recent advances in functional annotation and prediction of the epitranscriptome.Comput Struct Biotechnol J. 2021 May 21;19:3015-3026. doi: 10.1016/j.csbj.2021.05.030. eCollection 2021. Comput Struct Biotechnol J. 2021. PMID: 34136099 Free PMC article. Review.
References
-
- Cohn WE, Volkin E. Nucleoside-5′-phosphates from ribonucleic acid. Nature. 1951;167:483-484.
-
- Hamma T, Ferre-D’Amare AR. Pseudouridine synthases. Chem Biol. 2006;13:1125-1135. - PubMed
-
- McCleverty CJ, Hornsby M, Spraggon G, Kreusch A. Crystal structure of human Pus10, a novel pseudouridine synthase. J Mol Biol. 2007;373:1243-1254. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

