From biomechanics to mechanobiology: Xenopus provides direct access to the physical principles that shape the embryo
- PMID: 32563783
- PMCID: PMC9972463
- DOI: 10.1016/j.gde.2020.05.011
From biomechanics to mechanobiology: Xenopus provides direct access to the physical principles that shape the embryo
Abstract
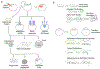
Features of amphibian embryos that have served so well to elucidate the genetics of vertebrate development also enable detailed analysis of the physics that shape morphogenesis and regulate development. Biophysical tools are revealing how genes control mechanical properties of the embryo. The same tools that describe and control mechanical properties are being turned to reveal how dynamic mechanical information and feedback regulate biological programs of development. In this review we outline efforts to explore the various roles of mechanical cues in guiding cilia biology, axonal pathfinding, goblet cell regeneration, epithelial-to-mesenchymal transitions in neural crest, and mesenchymal-to-epithelial transitions in heart progenitors. These case studies reveal the power of Xenopus experimental embryology to expose pathways integrating mechanical cues with programs of development, organogenesis, and regeneration.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors do not have any conflicts.
Figures
Similar articles
-
The Xenopus embryo as a model system for studies of cell migration.Methods Mol Biol. 2005;294:235-45. doi: 10.1385/1-59259-860-9:235. Methods Mol Biol. 2005. PMID: 15576916
-
Tissue stiffening coordinates morphogenesis by triggering collective cell migration in vivo.Nature. 2018 Feb 22;554(7693):523-527. doi: 10.1038/nature25742. Epub 2018 Feb 14. Nature. 2018. PMID: 29443958 Free PMC article.
-
AKT signaling displays multifaceted functions in neural crest development.Dev Biol. 2018 Dec 1;444 Suppl 1:S144-S155. doi: 10.1016/j.ydbio.2018.05.023. Epub 2018 May 31. Dev Biol. 2018. PMID: 29859890 Review.
-
Neural crest migration methods in the chicken embryo.Methods Mol Biol. 2005;294:247-67. doi: 10.1385/1-59259-860-9:247. Methods Mol Biol. 2005. PMID: 15576917
-
Emergent morphogenesis: elastic mechanics of a self-deforming tissue.J Biomech. 2010 Jan 5;43(1):63-70. doi: 10.1016/j.jbiomech.2009.09.010. Epub 2009 Oct 8. J Biomech. 2010. PMID: 19815213 Free PMC article. Review.
Cited by
-
The dynamics and biophysics of shape formation: Common themes in plant and animal morphogenesis.Dev Cell. 2023 Dec 18;58(24):2850-2866. doi: 10.1016/j.devcel.2023.11.003. Dev Cell. 2023. PMID: 38113851 Free PMC article. Review.
-
Adherens junctions as molecular regulators of emergent tissue mechanics.Nat Rev Mol Cell Biol. 2024 Apr;25(4):252-269. doi: 10.1038/s41580-023-00688-7. Epub 2023 Dec 13. Nat Rev Mol Cell Biol. 2024. PMID: 38093099 Review.
-
Screening Biophysical Sensors and Neurite Outgrowth Actuators in Human Induced-Pluripotent-Stem-Cell-Derived Neurons.Cells. 2022 Aug 9;11(16):2470. doi: 10.3390/cells11162470. Cells. 2022. PMID: 36010547 Free PMC article.
-
Development of a heat-stable alkaline phosphatase reporter system for cis-regulatory analysis and its application to 3D digital imaging of Xenopus embryonic tissues.Dev Growth Differ. 2024 Apr;66(3):256-265. doi: 10.1111/dgd.12919. Epub 2024 Mar 4. Dev Growth Differ. 2024. PMID: 38439617 Free PMC article.
-
Image-based parameter inference for epithelial mechanics.PLoS Comput Biol. 2022 Jun 23;18(6):e1010209. doi: 10.1371/journal.pcbi.1010209. eCollection 2022 Jun. PLoS Comput Biol. 2022. PMID: 35737656 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

