Cross-species reactivity of antibodies against Plasmodium vivax blood-stage antigens to Plasmodium knowlesi
- PMID: 32559186
- PMCID: PMC7304578
- DOI: 10.1371/journal.pntd.0008323
Cross-species reactivity of antibodies against Plasmodium vivax blood-stage antigens to Plasmodium knowlesi
Abstract
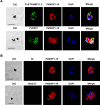
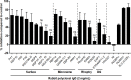
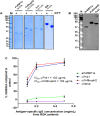
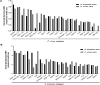
Malaria is caused by multiple different species of protozoan parasites, and interventions in the pre-elimination phase can lead to drastic changes in the proportion of each species causing malaria. In endemic areas, cross-reactivity may play an important role in the protection and blocking transmission. Thus, successful control of one species could lead to an increase in other parasite species. A few studies have reported cross-reactivity producing cross-immunity, but the extent of cross-reactive, particularly between closely related species, is poorly understood. P. vivax and P. knowlesi are particularly closely related species causing malaria infections in SE Asia, and whilst P. vivax cases are in decline, zoonotic P. knowlesi infections are rising in some areas. In this study, the cross-species reactivity and growth inhibition activity of P. vivax blood-stage antigen-specific antibodies against P. knowlesi parasites were investigated. Bioinformatics analysis, immunofluorescence assay, western blotting, protein microarray, and growth inhibition assay were performed to investigate the cross-reactivity. P. vivax blood-stage antigen-specific antibodies recognized the molecules located on the surface or released from apical organelles of P. knowlesi merozoites. Recombinant P. vivax and P. knowlesi proteins were also recognized by P. knowlesi- and P. vivax-infected patient antibodies, respectively. Immunoglobulin G against P. vivax antigens from both immune animals and human malaria patients inhibited the erythrocyte invasion by P. knowlesi. This study demonstrates that there is extensive cross-reactivity between antibodies against P. vivax to P. knowlesi in the blood stage, and these antibodies can potently inhibit in vitro invasion, highlighting the potential cross-protective immunity in endemic areas.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Cross-species analysis of apical asparagine-rich protein of Plasmodium vivax and Plasmodium knowlesi.Sci Rep. 2018 Apr 10;8(1):5781. doi: 10.1038/s41598-018-23728-1. Sci Rep. 2018. PMID: 29636493 Free PMC article.
-
Using Plasmodium knowlesi as a model for screening Plasmodium vivax blood-stage malaria vaccine targets reveals new candidates.PLoS Pathog. 2021 Jul 1;17(7):e1008864. doi: 10.1371/journal.ppat.1008864. eCollection 2021 Jul. PLoS Pathog. 2021. PMID: 34197567 Free PMC article.
-
A panel of recombinant proteins from human-infective Plasmodium species for serological surveillance.Malar J. 2020 Jan 17;19(1):31. doi: 10.1186/s12936-020-3111-5. Malar J. 2020. PMID: 31952523 Free PMC article.
-
Invasion of erythrocytes by malaria parasites: a cellular and molecular overview.Annu Rev Microbiol. 1986;40:451-77. doi: 10.1146/annurev.mi.40.100186.002315. Annu Rev Microbiol. 1986. PMID: 3535649 Review.
-
Immunity to sexual stages of human malaria parasites: immune modulation during natural infections, antigenic determinants, and the induction of transmission-blocking immunity.Scand J Infect Dis Suppl. 1990;76:79-88. Scand J Infect Dis Suppl. 1990. PMID: 1714627 Review.
Cited by
-
Determination of Plasmodium vivax and Plasmodium falciparum Malaria Exposure in Two Ethiopian Communities and Its Relationship to Duffy Expression.Am J Trop Med Hyg. 2023 Oct 16;109(5):1028-1035. doi: 10.4269/ajtmh.22-0644. Print 2023 Nov 1. Am J Trop Med Hyg. 2023. PMID: 37918005 Free PMC article.
-
Plasmodium knowlesi detection methods for human infections-Diagnosis and surveillance.Adv Parasitol. 2021;113:77-130. doi: 10.1016/bs.apar.2021.08.002. Epub 2021 Sep 17. Adv Parasitol. 2021. PMID: 34620386 Free PMC article.
-
Optimizing Plasmodium vivax serological surveillance within a coendemic epidemiological landscape.Trends Parasitol. 2022 Oct;38(10):829-830. doi: 10.1016/j.pt.2022.08.008. Epub 2022 Aug 26. Trends Parasitol. 2022. PMID: 36038428 Free PMC article.
-
Identification of conserved cross-species B-cell linear epitopes in human malaria: a subtractive proteomics and immuno-informatics approach targeting merozoite stage proteins.Front Immunol. 2024 Feb 9;15:1352618. doi: 10.3389/fimmu.2024.1352618. eCollection 2024. Front Immunol. 2024. PMID: 38404581 Free PMC article.
-
Plasmodium cynomolgi Berok Growth Inhibition Assay by Thiol-reactive Probe Based Flow Cytometric Measurement.Bio Protoc. 2021 Sep 5;11(17):e4147. doi: 10.21769/BioProtoc.4147. eCollection 2021 Sep 5. Bio Protoc. 2021. PMID: 34604452 Free PMC article.
References
-
- WHO. World malaria report 2017.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

