Mdm2-mediated neddylation of pVHL blocks the induction of antiangiogenic factors
- PMID: 32555333
- PMCID: PMC7368819
- DOI: 10.1038/s41388-020-1359-4
Mdm2-mediated neddylation of pVHL blocks the induction of antiangiogenic factors
Abstract
Mutations in the tumor suppressor TP53 are rare in renal cell carcinomas. p53 is a key factor for inducing antiangiogenic genes and RCC are highly vascularized, which suggests that p53 is inactive in these tumors. One regulator of p53 is the Mdm2 oncogene, which is correlated with high-grade, metastatic tumors. However, the sole activity of Mdm2 is not just to regulate p53, but it can also function independent of p53 to regulate the early stages of metastasis. Here, we report that the oncoprotein Mdm2 can bind directly to the tumor suppressor VHL, and conjugate nedd8 to VHL within a region that is important for the p53-VHL interaction. Nedd8 conjugated VHL is unable to bind to p53 thereby preventing the induction of antiangiogenic factors. These results highlight a previously unknown oncogenic function of Mdm2 during the progression of cancer to promote angiogenesis through the regulation of VHL. Thus, the Mdm2-VHL interaction represents a pathway that impacts tumor angiogenesis.
Conflict of interest statement
Competing Interests
The authors declare that they have no competing interests.
Figures
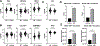
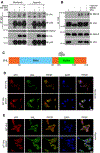

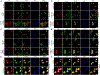
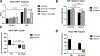
Similar articles
-
The von Hippel-Lindau tumor suppressor regulates programmed cell death 5-mediated degradation of Mdm2.Oncogene. 2015 Feb 5;34(6):771-9. doi: 10.1038/onc.2013.598. Epub 2014 Jan 27. Oncogene. 2015. PMID: 24469044
-
p53 stabilization and transactivation by a von Hippel-Lindau protein.Mol Cell. 2006 May 5;22(3):395-405. doi: 10.1016/j.molcel.2006.04.006. Mol Cell. 2006. PMID: 16678111
-
VHL missense mutations in the p53 binding domain show different effects on p53 signaling and HIFα degradation in clear cell renal cell carcinoma.Oncotarget. 2017 Feb 7;8(6):10199-10212. doi: 10.18632/oncotarget.14372. Oncotarget. 2017. PMID: 28052007 Free PMC article.
-
The positive regulation of p53 by the tumor suppressor VHL.Cell Cycle. 2006 Sep;5(18):2054-6. doi: 10.4161/cc.5.18.3247. Epub 2006 Sep 15. Cell Cycle. 2006. PMID: 16969113 Review.
-
The von hippel-lindau tumor suppressor protein: an update.Methods Enzymol. 2007;435:371-83. doi: 10.1016/S0076-6879(07)35019-2. Methods Enzymol. 2007. PMID: 17998064 Review.
Cited by
-
Induction of the Mdm2 gene and protein by kinase signaling pathways is repressed by the pVHL tumor suppressor.Proc Natl Acad Sci U S A. 2024 Jul 30;121(31):e2400935121. doi: 10.1073/pnas.2400935121. Epub 2024 Jul 24. Proc Natl Acad Sci U S A. 2024. PMID: 39047034 Free PMC article.
-
SHANK1 facilitates non-small cell lung cancer processes through modulating the ubiquitination of Klotho by interacting with MDM2.Cell Death Dis. 2022 Apr 25;13(4):403. doi: 10.1038/s41419-022-04860-3. Cell Death Dis. 2022. PMID: 35468874 Free PMC article.
-
FOXO1-regulated lncRNA CYP1B1-AS1 suppresses breast cancer cell proliferation by inhibiting neddylation.Breast Cancer Res Treat. 2023 Nov;202(2):397-408. doi: 10.1007/s10549-023-07090-z. Epub 2023 Aug 28. Breast Cancer Res Treat. 2023. PMID: 37640964 Free PMC article.
-
The E3 ubiquitin-protein ligase MDM2 is a novel interactor of the von Hippel-Lindau tumor suppressor.Sci Rep. 2020 Sep 28;10(1):15850. doi: 10.1038/s41598-020-72683-3. Sci Rep. 2020. PMID: 32985545 Free PMC article.
-
RA Fibroblast-Like Synoviocytes Derived Extracellular Vesicles Promote Angiogenesis by miRNA-1972 Targeting p53/mTOR Signaling in Vascular Endotheliocyte.Front Immunol. 2022 Mar 8;13:793855. doi: 10.3389/fimmu.2022.793855. eCollection 2022. Front Immunol. 2022. PMID: 35350778 Free PMC article.
References
-
- Rayburn E, Zhang R, He J, Wang H. MDM2 and human malignancies: expression, clinical pathology, prognostic markers, and implications for chemotherapy. Curr Cancer Drug Targets 2005; 5: 27–41. - PubMed
-
- Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature 1997; 387: 296–299. - PubMed
-
- Kubbutat MH, Jones SN, Vousden KH. Regulation of p53 stability by Mdm2. Nature 1997; 387: 299–303. - PubMed
-
- Miyashita T, Reed JC. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell 1995; 80: 293–299. - PubMed
-
- el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM et al. WAF1, a potential mediator of p53 tumor suppression. Cell 1993; 75: 817–825. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

