In vitro activity of the first-in-class triazaacenaphthylene gepotidacin alone and in combination with doxycycline against drug-resistant and -susceptible Mycoplasma genitalium
- PMID: 32552547
- PMCID: PMC7473033
- DOI: 10.1080/22221751.2020.1775498
In vitro activity of the first-in-class triazaacenaphthylene gepotidacin alone and in combination with doxycycline against drug-resistant and -susceptible Mycoplasma genitalium
Abstract
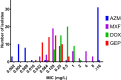
Mycoplasma genitalium has developed resistance to first-line azithromycin and second-line moxifloxacin. Third-line pristinamycin is only 75% effective. Gepotidacin, a novel triazaacenaphthylene topoisomerase II inhibitor, blocks bacterial DNA replication. We determined the in vitro activity of gepotidacin alone and in combination with doxycycline against a diverse collection of Mycoplasma genitalium isolates (n = 54). Minimum inhibitory concentrations (MICs) and minimum bactericidal concentrations (MBCs) were determined by a Vero-cell culture method. Macrolide resistance was present in 31 (57%) isolates, fluoroquinolone resistance in 18 (33%) isolates, and 17 (31%) had dual resistance. Synergy testing was performed for gepotidacin and doxycycline by checkerboard analysis for two macrolide- and two dual-resistant isolates. Gepotidacin was active against all 54 M. genitalium isolates with median and modal MICs of 0.125 mg/L and MIC90 of 0.25 mg/L (range ≤0.016-0.5 mg/L). No difference in gepotidacin MIC between macrolide-resistant and -susceptible isolates (p = 0.24) or between fluoroquinolone-, dual-resistant and -susceptible isolates (p = 0.2) was demonstrated. Gepotidacin MBCs were available for 44 M. genitalium isolates with median MIC of 0.064 mg/L and median MBC of 0.125 mg/L. All isolates had ≤4-fold difference between MIC and MBC, suggesting bactericidal effect for gepotidacin. Checkerboard analysis indicated synergistic effect for gepotidacin in combination with doxycycline [fractional inhibitory concentration index (ΣFICI) of 0.5] for two isolates and additive/indifference (ΣFICI at 0.62 and 0.75) for two isolates. Gepotidacin warrants further evaluation in clinical treatment trials for M. genitalium. Combination therapy with doxycycline should be clinically studied to assess effect and potential protection against development and/or spread of gepotidacin resistance.
Keywords: Mycoplasma genitalium; antimicrobial resistance; combination therapy; gepotidacin; in-vitro susceptibility testing.
Conflict of interest statement
JSJ has received speaker’s fee from Hologic, BD, SpeeDx, and Cepheid and serves scientific advisory board of Roche Molecular Systems, Abbott Molecular, Cepheid, and Nabriva. CHK and MU report no conflicts. NS-O is employed by GlaxoSmithKline.
Figures
Similar articles
-
In Vitro Activities of Gepotidacin (GSK2140944) and Other Antimicrobial Agents against Human Mycoplasmas and Ureaplasmas.Antimicrob Agents Chemother. 2017 Sep 22;61(10):e01064-17. doi: 10.1128/AAC.01064-17. Print 2017 Oct. Antimicrob Agents Chemother. 2017. PMID: 28784668 Free PMC article.
-
In vitro test of the novel antibiotic lefamulin alone and in combination with doxycycline against Mycoplasma genitalium.Antimicrob Agents Chemother. 2025 Jan 31;69(1):e0134624. doi: 10.1128/aac.01346-24. Epub 2024 Dec 13. Antimicrob Agents Chemother. 2025. PMID: 39670749 Free PMC article.
-
Gepotidacin (GSK2140944) In Vitro Activity against Gram-Positive and Gram-Negative Bacteria.Antimicrob Agents Chemother. 2017 Jun 27;61(7):e00468-17. doi: 10.1128/AAC.00468-17. Print 2017 Jul. Antimicrob Agents Chemother. 2017. PMID: 28483959 Free PMC article.
-
Efficacy of Antimicrobial Therapy for Mycoplasma genitalium Infections.Clin Infect Dis. 2015 Dec 15;61 Suppl 8:S802-17. doi: 10.1093/cid/civ785. Clin Infect Dis. 2015. PMID: 26602619 Review.
-
Molecular basis of antimicrobial resistance in Mycoplasma genitalium.Int J Antimicrob Agents. 2020 Apr;55(4):105911. doi: 10.1016/j.ijantimicag.2020.105911. Epub 2020 Jan 25. Int J Antimicrob Agents. 2020. PMID: 31991219 Review.
Cited by
-
Azithromycin and Doxycycline Resistance Profiles of U.S. Mycoplasma genitalium Strains and Their Association with Treatment Outcomes.J Clin Microbiol. 2021 Oct 19;59(11):e0081921. doi: 10.1128/JCM.00819-21. Epub 2021 Aug 18. J Clin Microbiol. 2021. PMID: 34406799 Free PMC article.
-
Antimicrobial treatment and resistance in sexually transmitted bacterial infections.Nat Rev Microbiol. 2024 Jul;22(7):435-450. doi: 10.1038/s41579-024-01023-3. Epub 2024 Mar 20. Nat Rev Microbiol. 2024. PMID: 38509173 Review.
-
Latest Advances in Laboratory Detection of Mycoplasma genitalium.J Clin Microbiol. 2023 Mar 23;61(3):e0079021. doi: 10.1128/jcm.00790-21. Epub 2023 Jan 4. J Clin Microbiol. 2023. PMID: 36598247 Free PMC article. Review.
-
Weighing Potential Benefits and Harms of Mycoplasma genitalium Testing and Treatment Approaches.Emerg Infect Dis. 2022 Aug;28(8):e220094. doi: 10.3201/eid2808.220094. Emerg Infect Dis. 2022. PMID: 35876565 Free PMC article.
-
Treatment of bacterial sexually transmitted infections in Europe: gonorrhoea, Mycoplasma genitalium, and syphilis.Lancet Reg Health Eur. 2023 Oct 26;34:100737. doi: 10.1016/j.lanepe.2023.100737. eCollection 2023 Nov. Lancet Reg Health Eur. 2023. PMID: 37927440 Free PMC article. Review.
References
-
- Sonnenberg P, Ison CA, Clifton S, et al. . Epidemiology of Mycoplasma genitalium in British men and women aged 16-44 years: evidence from the third National Survey of Sexual Attitudes and Lifestyles (Natsal-3). Int J Epidemiol. 2015. 12/2015;44(6):1982–1994. doi: 10.1093/ije/dyv194 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical