Innate immune recognition and modulation in hepatitis D virus infection
- PMID: 32550754
- PMCID: PMC7284172
- DOI: 10.3748/wjg.v26.i21.2781
Innate immune recognition and modulation in hepatitis D virus infection
Abstract
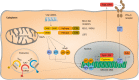
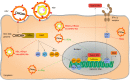
Hepatitis D virus (HDV) is a global health threat with more than 15 million humans affected. Current treatment options are largely unsatisfactory leaving chronically infected humans at high risk to develop liver cirrhosis and hepatocellular carcinoma. HDV is the only human satellite virus known. It encodes only two proteins, and requires Hepatitis B virus (HBV) envelope protein expression for productive virion release and spread of the infection. How HDV could evolve and why HBV was selected as a helper virus remains unknown. Since the discovery of Na+-taurocholate co-transporting polypeptide as the essential uptake receptor for HBV and HDV, we are beginning to understand the interactions of HDV and the immune system. While HBV is mostly regarded a stealth virus, that escapes innate immune recognition, HBV-HDV coinfection is characterized by a strong innate immune response. Cytoplasmic RNA sensor melanoma differentiation antigen 5 has been reported to recognize HDV RNA replication and activate innate immunity. Innate immunity, however, seems not to impair HDV replication while it inhibits HBV. In this review, we describe what is known up-to-date about the interplay between HBV as a helper and HDV's immune evasion strategy and identify where additional research is required.
Keywords: Hepatitis B virus; Hepatitis D virus; Immune evasion; Immunosuppression; Innate immunity; Pathogen-associated molecular pattern molecules.
©The Author(s) 2020. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: The authors declare no conflict of interests concerning the content of this article.
Figures
Similar articles
-
Hepatitis Delta co-infection in humanized mice leads to pronounced induction of innate immune responses in comparison to HBV mono-infection.J Hepatol. 2015 Aug;63(2):346-53. doi: 10.1016/j.jhep.2015.03.011. Epub 2015 Mar 17. J Hepatol. 2015. PMID: 25795587
-
Interplay between Hepatitis D Virus and the Interferon Response.Viruses. 2020 Nov 20;12(11):1334. doi: 10.3390/v12111334. Viruses. 2020. PMID: 33233762 Free PMC article. Review.
-
A new HDV mouse model identifies mitochondrial antiviral signaling protein (MAVS) as a key player in IFN-β induction.J Hepatol. 2017 Oct;67(4):669-679. doi: 10.1016/j.jhep.2017.05.010. Epub 2017 May 18. J Hepatol. 2017. PMID: 28527664
-
Primary biliary acids inhibit hepatitis D virus (HDV) entry into human hepatoma cells expressing the sodium-taurocholate cotransporting polypeptide (NTCP).PLoS One. 2015 Feb 3;10(2):e0117152. doi: 10.1371/journal.pone.0117152. eCollection 2015. PLoS One. 2015. PMID: 25646622 Free PMC article.
-
HDV Pathogenesis: Unravelling Ariadne's Thread.Viruses. 2021 Apr 28;13(5):778. doi: 10.3390/v13050778. Viruses. 2021. PMID: 33924806 Free PMC article. Review.
Cited by
-
Many Ways to Communicate-Crosstalk between the HBV-Infected Cell and Its Environment.Pathogens. 2022 Dec 24;12(1):29. doi: 10.3390/pathogens12010029. Pathogens. 2022. PMID: 36678377 Free PMC article. Review.
-
Hepatitis B and Hepatitis D Viruses: A Comprehensive Update with an Immunological Focus.Int J Mol Sci. 2022 Dec 15;23(24):15973. doi: 10.3390/ijms232415973. Int J Mol Sci. 2022. PMID: 36555623 Free PMC article. Review.
-
The Use of Molecular Dynamics Simulation Method to Quantitatively Evaluate the Affinity between HBV Antigen T Cell Epitope Peptides and HLA-A Molecules.Int J Mol Sci. 2022 Apr 22;23(9):4629. doi: 10.3390/ijms23094629. Int J Mol Sci. 2022. PMID: 35563019 Free PMC article.
-
Efficient and reproducible depletion of hepatitis B virus from plasma derived extracellular vesicles.J Extracell Vesicles. 2020 Dec;10(2):e12040. doi: 10.1002/jev2.12040. Epub 2020 Dec 22. J Extracell Vesicles. 2020. PMID: 33363711 Free PMC article.
-
Signaling Induced by Chronic Viral Hepatitis: Dependence and Consequences.Int J Mol Sci. 2022 Mar 3;23(5):2787. doi: 10.3390/ijms23052787. Int J Mol Sci. 2022. PMID: 35269929 Free PMC article. Review.
References
-
- Magnius L, Taylor J, Mason WS, Sureau C, Dény P, Norder H, Ictv Report Consortium. ICTV Virus Taxonomy Profile: Deltavirus. J Gen Virol. 2018;99:1565–1566. - PubMed
-
- World Health Organization. Hepatitis D, 2019. Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-d.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

