A 1-year prospective, open-label, single-arm, multicenter, phase 3 trial of the contraceptive efficacy and safety of the oral progestin-only pill drospirenone 4 mg using a 24/4-day regimen
- PMID: 32550535
- PMCID: PMC7286157
- DOI: 10.1016/j.conx.2020.100020
A 1-year prospective, open-label, single-arm, multicenter, phase 3 trial of the contraceptive efficacy and safety of the oral progestin-only pill drospirenone 4 mg using a 24/4-day regimen
Abstract
Objectives: To evaluate contraceptive effectiveness and safety of oral drospirenone 4 mg 24/4-day regimen in the United States.
Study design: We performed a prospective, single-arm, multicenter phase 3 trial in sexually active women for up to thirteen 28-day treatment cycles. Primary outcome was the Pearl index, calculated using confirmed on-drug pregnancies and evaluable cycles in nonbreastfeeding women aged ≤ 35 years. We assessed adverse events (AEs), including hyperkalemia and venous thromboembolism.
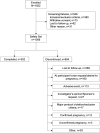
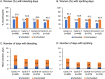
Results: Of 1006 women who received at least one dose of drospirenone, 352 women (35.0%) completed the trial and 654 (65.0%) women discontinued before trial end. Most participants (92.2%) were ≤ 35 years; one third had a body mass index (BMI) ≥ 30 kg/m2. Among nonbreastfeeding women aged ≤ 35 years, there were 17 pregnancies (Pearl index: 4.0; 95% confidence interval [CI], 2.3-6.4; n = 953), of which three were unconfirmed and two were from sites excluded from the main analysis for major breaches of Food and Drug Administration regulations. The Pearl index was 2.9 (95% CI: 1.5-5.1) for confirmed pregnancies among 915 nonbreastfeeding women aged ≤ 35 years from sites with no protocol violations. Nearly all (95.4%) treatment-emergent AEs were mild or moderate in intensity. No cases of venous thromboembolism were reported. The frequency of hyperkalemia was 0.5%. Women with baseline systolic/diastolic blood pressure ≥ 130/85 mmHg had a mean reduction from baseline in blood pressure at exit visit (- 8.5/- 4.9 mmHg; n = 119). No other clinically relevant changes were observed. Participant satisfaction was high.
Conclusion: Drospirenone 4 mg 24/4 regimen provides effective contraception with a good safety/tolerability profile in a broad group of women, including overweight or obese women.
Implications: This new progestin-only contraceptive, drospirenone 4 mg in a 24/4 regimen, provides a contraceptive option for the majority of women regardless of blood pressure or BMI.
Keywords: 24/4-day regimen; Efficacy; Progesterone/progestin-only pill; Safety; Scheduled bleeding.
© 2020 The Authors.
Figures
Similar articles
-
The efficacy, safety, and tolerability of an estrogen-free oral contraceptive drospirenone 4 mg (24/4-day regimen) in obese users.Contraception. 2023 Dec;128:110136. doi: 10.1016/j.contraception.2023.110136. Epub 2023 Aug 5. Contraception. 2023. PMID: 37544572
-
Multicenter, phase III trials on the contraceptive efficacy, tolerability and safety of a new drospirenone-only pill.Acta Obstet Gynecol Scand. 2019 Dec;98(12):1549-1557. doi: 10.1111/aogs.13688. Epub 2019 Aug 6. Acta Obstet Gynecol Scand. 2019. PMID: 31321765 Free PMC article. Clinical Trial.
-
Estetrol-Drospirenone combination oral contraceptive: a clinical study of contraceptive efficacy, bleeding pattern and safety in Europe and Russia.BJOG. 2022 Jan;129(1):63-71. doi: 10.1111/1471-0528.16840. Epub 2021 Aug 9. BJOG. 2022. PMID: 34245666 Free PMC article. Clinical Trial.
-
[Individualization of low-dose oral contraceptives. Pharmacological principles and practical indications for oral contraceptives].Minerva Ginecol. 2007 Aug;59(4):415-25. Minerva Ginecol. 2007. PMID: 17923832 Review. Italian.
-
Drospirenone in combination with estrogens: for contraception and hormone replacement therapy.Climacteric. 2005 Oct;8 Suppl 3:19-27. doi: 10.1080/13697130500330341. Climacteric. 2005. PMID: 16203652 Review.
Cited by
-
Safety of Progestogen Hormonal Contraceptive Methods during Lactation: An Overview.Clin Pract. 2024 Jun 4;14(3):1054-1064. doi: 10.3390/clinpract14030083. Clin Pract. 2024. PMID: 38921261 Free PMC article. Review.
-
Efficacy and cardiovascular safety of the new estrogen-free contraceptive pill containing 4 mg drospirenone alone in a 24/4 regime.BMC Womens Health. 2020 Oct 2;20(1):218. doi: 10.1186/s12905-020-01080-9. BMC Womens Health. 2020. PMID: 33008401 Free PMC article. Clinical Trial.
-
Evaluation of the food effect on a drospirenone only contraceptive containing 4 mg administered with and without high-fat breakfast in a randomised trial.BMC Womens Health. 2022 Sep 19;22(1):381. doi: 10.1186/s12905-022-01960-2. BMC Womens Health. 2022. PMID: 36123682 Free PMC article. Clinical Trial.
-
Oral Contraceptive Pills and Hypertension: A Review of Current Evidence and Recommendations.Hypertension. 2023 May;80(5):924-935. doi: 10.1161/HYPERTENSIONAHA.122.20018. Epub 2023 Feb 16. Hypertension. 2023. PMID: 37075131 Free PMC article. Review.
-
Bleeding Patterns of Oral Contraceptives with a Cyclic Dosing Regimen: An Overview.J Clin Med. 2022 Aug 8;11(15):4634. doi: 10.3390/jcm11154634. J Clin Med. 2022. PMID: 35956249 Free PMC article. Review.
References
-
- Shortridge E., Miller K. Contraindications to oral contraceptive use among women in the United States, 1999-2001. Contraception. 2007;75:355–360. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

