Interplay Between SNX27 and DAG Metabolism in the Control of Trafficking and Signaling at the IS
- PMID: 32549284
- PMCID: PMC7352468
- DOI: 10.3390/ijms21124254
Interplay Between SNX27 and DAG Metabolism in the Control of Trafficking and Signaling at the IS
Abstract
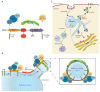
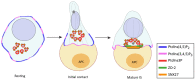
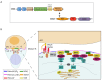
Recognition of antigens displayed on the surface of an antigen-presenting cell (APC) by T-cell receptors (TCR) of a T lymphocyte leads to the formation of a specialized contact between both cells named the immune synapse (IS). This highly organized structure ensures cell-cell communication and sustained T-cell activation. An essential lipid regulating T-cell activation is diacylglycerol (DAG), which accumulates at the cell-cell interface and mediates recruitment and activation of proteins involved in signaling and polarization. Formation of the IS requires rearrangement of the cytoskeleton, translocation of the microtubule-organizing center (MTOC) and vesicular compartments, and reorganization of signaling and adhesion molecules within the cell-cell junction. Among the multiple players involved in this polarized intracellular trafficking, we find sorting nexin 27 (SNX27). This protein translocates to the T cell-APC interface upon TCR activation, and it is suggested to facilitate the transport of cargoes toward this structure. Furthermore, its interaction with diacylglycerol kinase ζ (DGKζ), a negative regulator of DAG, sustains the precise modulation of this lipid and, thus, facilitates IS organization and signaling. Here, we review the role of SNX27, DAG metabolism, and their interplay in the control of T-cell activation and establishment of the IS.
Keywords: SNX27; diacylglycerol; diacylglycerol kinase; immune synapse; intracellular trafficking; retromer.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Sorting Nexin 27 Enables MTOC and Secretory Machinery Translocation to the Immune Synapse.Front Immunol. 2022 Jan 12;12:814570. doi: 10.3389/fimmu.2021.814570. eCollection 2021. Front Immunol. 2022. PMID: 35095913 Free PMC article.
-
Translocation dynamics of sorting nexin 27 in activated T cells.J Cell Sci. 2011 Mar 1;124(Pt 5):776-88. doi: 10.1242/jcs.072447. Epub 2011 Feb 8. J Cell Sci. 2011. PMID: 21303929
-
SNX27 links DGKζ to the control of transcriptional and metabolic programs in T lymphocytes.Sci Rep. 2017 Nov 27;7(1):16361. doi: 10.1038/s41598-017-16370-w. Sci Rep. 2017. PMID: 29180720 Free PMC article.
-
Diacylglycerol kinase control of protein kinase C.Biochem J. 2019 Apr 18;476(8):1205-1219. doi: 10.1042/BCJ20180620. Biochem J. 2019. PMID: 31000624 Review.
-
Regulation of microtubule-organizing center orientation and actomyosin cytoskeleton rearrangement during immune interactions.Immunol Rev. 2002 Nov;189:84-97. doi: 10.1034/j.1600-065x.2002.18908.x. Immunol Rev. 2002. PMID: 12445267 Review.
Cited by
-
F-Actin Dynamics in the Regulation of Endosomal Recycling and Immune Synapse Assembly.Front Cell Dev Biol. 2021 Jun 24;9:670882. doi: 10.3389/fcell.2021.670882. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34249926 Free PMC article. Review.
-
SNX27-FERM-SNX1 complex structure rationalizes divergent trafficking pathways by SNX17 and SNX27.Proc Natl Acad Sci U S A. 2021 Sep 7;118(36):e2105510118. doi: 10.1073/pnas.2105510118. Proc Natl Acad Sci U S A. 2021. PMID: 34462354 Free PMC article.
-
The Regulation Network of Glycerolipid Metabolism as Coregulators of Immunotherapy-Related Myocarditis.Cardiovasc Ther. 2023 Jun 21;2023:8774971. doi: 10.1155/2023/8774971. eCollection 2023. Cardiovasc Ther. 2023. PMID: 37388276 Free PMC article.
-
DGKα, Bridging Membrane Shape Changes with Specific Molecular Species of DAG/PA: Implications in Cancer and Immunosurveillance.Cancers (Basel). 2022 Oct 26;14(21):5259. doi: 10.3390/cancers14215259. Cancers (Basel). 2022. PMID: 36358678 Free PMC article. Review.
References
-
- Alcover A., Thoulouze M.-I. Vesicle Traffic to the Immunological Synapse: A Multifunctional Process Targeted by Lymphotropic Viruses. In: Saito T., Batista F.D., editors. Immunological Synapse. Vol. 340. Springer Berlin Heidelberg; Berlin/Heidelberg, Germany: 2010. pp. 191–207. Current Topics in Microbiology and Immunology. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AECC- CICPF18/Spanish Association against Cancer
- LCF/BQ/DI17/11620027/Marie Sklodowska-Curie and La Caixa Foundatin
- OPE01644/Aplastic Anemia and MDS International Foundation
- BFU2016-77207-R/Ministerio Español de Economia y Competitividad
- IMMUNOTHERCAM S2010/BMD-2326/Consejería de Educación e Investigación

