TRPA1 and TRPV1 channels participate in atmospheric-pressure plasma-induced [Ca2+]i response
- PMID: 32546738
- PMCID: PMC7297720
- DOI: 10.1038/s41598-020-66510-y
TRPA1 and TRPV1 channels participate in atmospheric-pressure plasma-induced [Ca2+]i response
Abstract
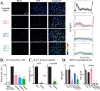
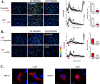
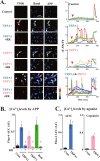
Despite successful clinical application of non-equilibrium atmospheric pressure plasma (APP), the details of the molecular mechanisms underlying APP-inducible biological responses remain ill-defined. We previously reported that exposure of 3T3L1 cells to APP-irradiated buffer raised the cytoplasmic free Ca2+ ([Ca2+]i) concentration by eliciting Ca2+ influx in a manner sensitive to transient receptor potential (TRP) channel inhibitors. However, the precise identity of the APP-responsive channel molecule(s) remains unclear. In the present study, we aimed to clarify channel molecule(s) responsible for indirect APP-responsive [Ca2+]i rises. siRNA-mediated silencing experiments revealed that TRPA1 and TRPV1 serve as the major APP-responsive Ca2+ channels in 3T3L1 cells. Conversely, ectopic expression of either TRPA1 or TRPV1 in APP-unresponsive C2C12 cells actually triggered [Ca2+]i elevation in response to indirect APP exposure. Desensitization experiments using 3T3L1 cells revealed APP responsiveness to be markedly suppressed after pretreatment with allyl isothiocyanate or capsaicin, TRPA1 and TRPV1 agonists, respectively. APP exposure also desensitized the cells to these chemical agonists, indicating the existence of a bi-directional heterologous desensitization property of APP-responsive [Ca2+]i transients mediated through these TRP channels. Mutational analyses of key cysteine residues in TRPA1 (Cys421, Cys621, Cys641, and Cys665) and in TRPV1 (Cys258, Cys363, and Cys742) have suggested that multiple reactive oxygen and nitrogen species are intricately involved in activation of the channels via a broad range of modifications involving these cysteine residues. Taken together, these observations allow us to conclude that both TRPA1 and TRPV1 channels play a pivotal role in evoking indirect APP-dependent [Ca2+]i responses.
Conflict of interest statement
The authors declare no competing interests.
Figures
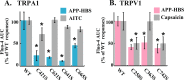
Similar articles
-
Identification of molecular targets for toxic action by persulfate, an industrial sulfur compound.Neurotoxicology. 2019 May;72:29-37. doi: 10.1016/j.neuro.2019.02.003. Epub 2019 Feb 6. Neurotoxicology. 2019. PMID: 30738091
-
Antimycin A-induced mitochondrial dysfunction activates vagal sensory neurons via ROS-dependent activation of TRPA1 and ROS-independent activation of TRPV1.Brain Res. 2019 Jul 15;1715:94-105. doi: 10.1016/j.brainres.2019.03.029. Epub 2019 Mar 23. Brain Res. 2019. PMID: 30914247 Free PMC article.
-
Functional Transient Receptor Potential Ankyrin 1 and Vanilloid 1 Ion Channels Are Overexpressed in Human Oral Squamous Cell Carcinoma.Int J Mol Sci. 2022 Feb 8;23(3):1921. doi: 10.3390/ijms23031921. Int J Mol Sci. 2022. PMID: 35163843 Free PMC article.
-
TRP channels in cancer pain.Eur J Pharmacol. 2021 Aug 5;904:174185. doi: 10.1016/j.ejphar.2021.174185. Epub 2021 May 17. Eur J Pharmacol. 2021. PMID: 34015320 Review.
-
[Activation and regulation of nociceptive transient receptor potential (TRP) channels, TRPV1 and TRPA1].Yakugaku Zasshi. 2010 Mar;130(3):289-94. doi: 10.1248/yakushi.130.289. Yakugaku Zasshi. 2010. PMID: 20190512 Review. Japanese.
Cited by
-
Sensory Nutrition and Bitterness and Astringency of Polyphenols.Biomolecules. 2024 Feb 17;14(2):234. doi: 10.3390/biom14020234. Biomolecules. 2024. PMID: 38397471 Free PMC article. Review.
-
Biochemical and Physiological Plant Processes Affected by Seed Treatment with Non-Thermal Plasma.Plants (Basel). 2022 Mar 23;11(7):856. doi: 10.3390/plants11070856. Plants (Basel). 2022. PMID: 35406836 Free PMC article. Review.
References
-
- Kaneko, T., Sasaki, S., Takashima, K. & Kanzaki, M. Gas-liquid interfacial plasmas producing reactive species for cell membrane permeabilization. J. Clin. Biochem. Nutr.60, 3–11, https://doi.org/10.3164/jcbn 16–73 (2017). - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

