CCR5-Mediated Signaling Is Involved in Invasion of Glioblastoma Cells in Its Microenvironment
- PMID: 32545571
- PMCID: PMC7352708
- DOI: 10.3390/ijms21124199
CCR5-Mediated Signaling Is Involved in Invasion of Glioblastoma Cells in Its Microenvironment
Abstract
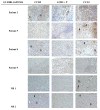
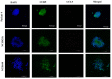
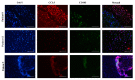
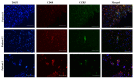
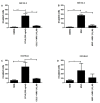
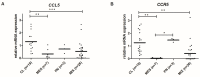
The chemokine CCL5/RANTES is a versatile inflammatory mediator, which interacts with the receptor CCR5, promoting cancer cell interactions within the tumor microenvironment. Glioblastoma is a highly invasive tumor, in which CCL5 expression correlates with shorter patient survival. Using immunohistochemistry, we identified CCL5 and CCR5 in a series of glioblastoma samples and cells, including glioblastoma stem cells. CCL5 and CCR5 gene expression were significantly higher in a cohort of 38 glioblastoma samples, compared to low-grade glioma and non-cancerous tissues. The in vitro invasion of patients-derived primary glioblastoma cells and glioblastoma stem cells was dependent on CCL5-induced CCR5 signaling and is strongly inhibited by the small molecule CCR5 antagonist maraviroc. Invasion of these cells, which was enhanced when co-cultured with mesenchymal stem cells (MSCs), was inhibited by maraviroc, suggesting that MSCs release CCR5 ligands. In support of this model, we detected CCL5 and CCR5 in MSC monocultures and glioblastoma-associated MSC in tissue sections. We also found CCR5 expressing macrophages were in close proximity to glioblastoma cells. In conclusion, autocrine and paracrine cross-talk in glioblastoma and, in particular, glioblastoma stem cells with its stromal microenvironment, involves CCR5 and CCL5, contributing to glioblastoma invasion, suggesting the CCL5/CCR5 axis as a potential therapeutic target that can be targeted with repositioned drug maraviroc.
Keywords: CCL5; CCR5; chemokines; glioblastoma; invasion; maraviroc; mesenchymal stem cells.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures


Similar articles
-
Cytokine CCL5 and receptor CCR5 axis in glioblastoma multiforme.Radiol Oncol. 2019 Nov 20;53(4):397-406. doi: 10.2478/raon-2019-0057. Radiol Oncol. 2019. PMID: 31747383 Free PMC article. Review.
-
Critical roles of chemokine receptor CCR5 in regulating glioblastoma proliferation and invasion.Acta Biochim Biophys Sin (Shanghai). 2015 Nov;47(11):890-8. doi: 10.1093/abbs/gmv095. Epub 2015 Sep 20. Acta Biochim Biophys Sin (Shanghai). 2015. PMID: 26390883
-
CCR5 antagonism by maraviroc inhibits Hodgkin lymphoma microenvironment interactions and xenograft growth.Haematologica. 2019 Mar;104(3):564-575. doi: 10.3324/haematol.2018.196725. Epub 2018 Oct 11. Haematologica. 2019. PMID: 30309853 Free PMC article.
-
Ccl5 establishes an autocrine high-grade glioma growth regulatory circuit critical for mesenchymal glioblastoma survival.Oncotarget. 2017 May 16;8(20):32977-32989. doi: 10.18632/oncotarget.16516. Oncotarget. 2017. PMID: 28380429 Free PMC article.
-
An Update on Glioblastoma Biology, Genetics, and Current Therapies: Novel Inhibitors of the G Protein-Coupled Receptor CCR5.Int J Mol Sci. 2021 Apr 24;22(9):4464. doi: 10.3390/ijms22094464. Int J Mol Sci. 2021. PMID: 33923334 Free PMC article. Review.
Cited by
-
Tumor-associated macrophages: an effective player of the tumor microenvironment.Front Immunol. 2023 Nov 16;14:1295257. doi: 10.3389/fimmu.2023.1295257. eCollection 2023. Front Immunol. 2023. PMID: 38035101 Free PMC article. Review.
-
The Role and Therapeutic Targeting of CCR5 in Breast Cancer.Cells. 2023 Sep 8;12(18):2237. doi: 10.3390/cells12182237. Cells. 2023. PMID: 37759462 Free PMC article. Review.
-
A Pyroptosis-Related Gene Signature Associated with Prognosis and Tumor Immune Microenvironment in Gliomas.Int J Gen Med. 2022 May 6;15:4753-4769. doi: 10.2147/IJGM.S353762. eCollection 2022. Int J Gen Med. 2022. PMID: 35571289 Free PMC article.
-
Biallelic, Selectable, Knock-in Targeting of CCR5 via CRISPR-Cas9 Mediated Homology Directed Repair Inhibits HIV-1 Replication.Front Immunol. 2022 Mar 21;13:821190. doi: 10.3389/fimmu.2022.821190. eCollection 2022. Front Immunol. 2022. PMID: 35386712 Free PMC article.
-
The Basis and Advances in Clinical Application of Cytomegalovirus-Specific Cytotoxic T Cell Immunotherapy for Glioblastoma Multiforme.Front Oncol. 2022 Apr 19;12:818447. doi: 10.3389/fonc.2022.818447. eCollection 2022. Front Oncol. 2022. PMID: 35515137 Free PMC article. Review.
References
-
- Louis D.N., Perry A., Reifenberger G., von Deimling A., Figarella-Branger D., Cavenee W.K., Ohgaki H., Wiestler O.D., Kleihues P., Ellison D.W. The 2016 world health organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

