Antibiotic-Induced Changes in Microbiome-Related Metabolites and Bile Acids in Rat Plasma
- PMID: 32545183
- PMCID: PMC7344402
- DOI: 10.3390/metabo10060242
Antibiotic-Induced Changes in Microbiome-Related Metabolites and Bile Acids in Rat Plasma
Abstract
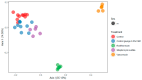
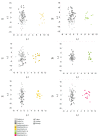
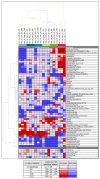
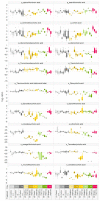
Various environmental factors can alter the gut microbiome's composition and functionality, and modulate host health. In this study, the effects of oral and parenteral administration of two poorly bioavailable antibiotics (i.e., vancomycin and streptomycin) on male Wistar Crl/Wi(Han) rats for 28 days were compared to distinguish between microbiome-derived or -associated and systemic changes in the plasma metabolome. The resulting changes in the plasma metabolome were compared to the effects of a third reference compound, roxithromycin, which is readily bioavailable. A community analysis revealed that the oral administration of vancomycin and roxithromycin in particular leads to an altered microbial population. Antibiotic-induced changes depending on the administration routes were observed in plasma metabolite levels. Indole-3-acetic acid (IAA) and hippuric acid (HA) were identified as key metabolites of microbiome modulation, with HA being the most sensitive. Even though large variations in the plasma bile acid pool between and within rats were observed, the change in microbiome community was observed to alter the composition of the bile acid pool, especially by an accumulation of taurine-conjugated primary bile acids. In-depth investigation of the relationship between microbiome variability and their functionality, with emphasis on the bile acid pool, will be necessary to better assess the potential adverseness of environmentally induced microbiome changes.
Keywords: antibiotics; bile acids; metabolomics; microbiome.
Conflict of interest statement
The authors declare no conflict of interest, there is no connection between BASF SE and the subject of this manuscript.
Figures
Similar articles
-
Gut Microbiota as Well as Metabolomes of Wistar Rats Recover within Two Weeks after Doripenem Antibiotic Treatment.Microorganisms. 2023 Feb 20;11(2):533. doi: 10.3390/microorganisms11020533. Microorganisms. 2023. PMID: 36838498 Free PMC article.
-
Analysis of metabolome changes in the bile acid pool in feces and plasma of antibiotic-treated rats.Toxicol Appl Pharmacol. 2019 Jan 15;363:79-87. doi: 10.1016/j.taap.2018.11.012. Epub 2018 Nov 28. Toxicol Appl Pharmacol. 2019. PMID: 30502395
-
Gut microbiome-related metabolic changes in plasma of antibiotic-treated rats.Arch Toxicol. 2017 Oct;91(10):3439-3454. doi: 10.1007/s00204-017-1949-2. Epub 2017 Mar 23. Arch Toxicol. 2017. PMID: 28337503
-
Rethinking the bile acid/gut microbiome axis in cancer.Oncotarget. 2017 Dec 1;8(70):115736-115747. doi: 10.18632/oncotarget.22803. eCollection 2017 Dec 29. Oncotarget. 2017. PMID: 29383197 Free PMC article. Review.
-
The human gut sterolbiome: bile acid-microbiome endocrine aspects and therapeutics.Acta Pharm Sin B. 2015 Mar;5(2):99-105. doi: 10.1016/j.apsb.2015.01.006. Epub 2015 Feb 9. Acta Pharm Sin B. 2015. PMID: 26579434 Free PMC article. Review.
Cited by
-
Bile acid metabolism and signaling in health and disease: molecular mechanisms and therapeutic targets.Signal Transduct Target Ther. 2024 Apr 26;9(1):97. doi: 10.1038/s41392-024-01811-6. Signal Transduct Target Ther. 2024. PMID: 38664391 Free PMC article. Review.
-
Gut Microbiota as Well as Metabolomes of Wistar Rats Recover within Two Weeks after Doripenem Antibiotic Treatment.Microorganisms. 2023 Feb 20;11(2):533. doi: 10.3390/microorganisms11020533. Microorganisms. 2023. PMID: 36838498 Free PMC article.
-
MICROBIOME: The trials and errors of developing an experimental model to study the impact of maternal gut microbiome disruption on perinatal asphyxia.Reprod Fertil. 2024 Nov 6;5(4):e240050. doi: 10.1530/RAF-24-0050. Print 2024 Oct 1. Reprod Fertil. 2024. PMID: 39374132 Free PMC article.
References
LinkOut - more resources
Full Text Sources

