Cross talk between mesenchymal and glioblastoma stem cells: Communication beyond controversies
- PMID: 32543030
- PMCID: PMC7581451
- DOI: 10.1002/sctm.20-0161
Cross talk between mesenchymal and glioblastoma stem cells: Communication beyond controversies
Abstract
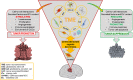
Mesenchymal stem cells (MSCs) can be isolated from bone marrow or other adult tissues (adipose tissue, dental pulp, amniotic fluid, and umbilical cord). In vitro, MSCs grow as adherent cells, display fibroblast-like morphology, and self-renew, undergoing specific mesodermal differentiation. High heterogeneity of MSCs from different origin, and differences in preparation techniques, make difficult to uniform their functional properties for therapeutic purposes. Immunomodulatory, migratory, and differentiation ability, fueled clinical MSC application in regenerative medicine, whereas beneficial effects are currently mainly ascribed to their secretome and extracellular vesicles. MSC translational potential in cancer therapy exploits putative anti-tumor activity and inherent tropism toward tumor sites to deliver cytotoxic drugs. However, controversial results emerged evaluating either the therapeutic potential or homing efficiency of MSCs, as both antitumor and protumor effects were reported. Glioblastoma (GBM) is the most malignant brain tumor and its development and aggressive nature is sustained by cancer stem cells (CSCs) and the identification of effective therapeutic is required. MSC dualistic action, tumor-promoting or tumor-targeting, is dependent on secreted factors and extracellular vesicles driving a complex cross talk between MSCs and GBM CSCs. Tumor-tropic ability of MSCs, besides providing an alternative therapeutic approach, could represent a tool to understand the biology of GBM CSCs and related paracrine mechanisms, underpinning MSC-GBM interactions. In this review, recent findings on the complex nature of MSCs will be highlighted, focusing on their elusive impact on GBM progression and aggressiveness by direct cell-cell interaction and via secretome, also facing the perspectives and challenges in treatment strategies.
Keywords: cancer stem cells; extracellular vesicles; glioblastoma; mesenchymal stem cells; secretoma.
© 2020 The Authors. STEM CELLS TRANSLATIONAL MEDICINE published by Wiley Periodicals LLC on behalf of AlphaMed Press.
Conflict of interest statement
The authors declared no potential conflicts of interest.
Figures
Similar articles
-
Mesenchymal stem cells in glioblastoma therapy and progression: How one cell does it all.Biochim Biophys Acta Rev Cancer. 2021 Aug;1876(1):188582. doi: 10.1016/j.bbcan.2021.188582. Epub 2021 Jun 16. Biochim Biophys Acta Rev Cancer. 2021. PMID: 34144129 Review.
-
Tropism of mesenchymal stem cell toward CD133+ stem cell of glioblastoma in vitro and promote tumor proliferation in vivo.Stem Cell Res Ther. 2018 Nov 9;9(1):310. doi: 10.1186/s13287-018-1049-0. Stem Cell Res Ther. 2018. PMID: 30413179 Free PMC article.
-
High Adenosine Extracellular Levels Induce Glioblastoma Aggressive Traits Modulating the Mesenchymal Stromal Cell Secretome.Int J Mol Sci. 2020 Oct 18;21(20):7706. doi: 10.3390/ijms21207706. Int J Mol Sci. 2020. PMID: 33081024 Free PMC article.
-
Umbilical cord blood-derived mesenchymal stem cells inhibit, but adipose tissue-derived mesenchymal stem cells promote, glioblastoma multiforme proliferation.Stem Cells Dev. 2013 May 1;22(9):1370-86. doi: 10.1089/scd.2012.0486. Epub 2013 Feb 4. Stem Cells Dev. 2013. PMID: 23231075 Free PMC article.
-
Cytokines play a key role in communication between mesenchymal stem cells and brain cancer cells.Protein Pept Lett. 2015;22(4):322-31. doi: 10.2174/0929866522666150131123808. Protein Pept Lett. 2015. PMID: 25642990 Review.
Cited by
-
Non-Tumor Cells within the Tumor Microenvironment-The "Eminence Grise" of the Glioblastoma Pathogenesis and Potential Targets for Therapy.Cells. 2024 May 9;13(10):808. doi: 10.3390/cells13100808. Cells. 2024. PMID: 38786032 Free PMC article. Review.
-
Stem cells for the treatment of glioblastoma: a 20-year perspective.CNS Oncol. 2021 Jun 1;10(2):CNS73. doi: 10.2217/cns-2020-0026. Epub 2021 May 19. CNS Oncol. 2021. PMID: 34006134 Free PMC article. Review.
-
Mesenchymal-Stem-Cell-Based Therapy against Gliomas.Cells. 2024 Apr 2;13(7):617. doi: 10.3390/cells13070617. Cells. 2024. PMID: 38607056 Free PMC article. Review.
-
Cadherin-6 is a novel mediator for the migration of mesenchymal stem cells to glioblastoma cells in response to stromal cell-derived factor-1.FEBS Open Bio. 2024 Jul;14(7):1192-1204. doi: 10.1002/2211-5463.13815. Epub 2024 May 8. FEBS Open Bio. 2024. PMID: 38719785 Free PMC article.
-
Pharmacokinetic characteristics of mesenchymal stem cells in translational challenges.Signal Transduct Target Ther. 2024 Sep 13;9(1):242. doi: 10.1038/s41392-024-01936-8. Signal Transduct Target Ther. 2024. PMID: 39271680 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

