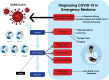
Diagnosing COVID-19 in the Emergency Department: A Scoping Review of Clinical Examinations, Laboratory Tests, Imaging Accuracy, and Biases
- PMID: 32542934
- PMCID: PMC7323136
- DOI: 10.1111/acem.14048
Diagnosing COVID-19 in the Emergency Department: A Scoping Review of Clinical Examinations, Laboratory Tests, Imaging Accuracy, and Biases
Abstract
Objective: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged as a global pandemic in early 2020 with rapidly evolving approaches to diagnosing the clinical illness called coronavirus disease (COVID-19). The primary objective of this scoping review is to synthesize current research of the diagnostic accuracy of history, physical examination, routine laboratory tests, real-time reverse transcription-polymerase chain reaction (rRT-PCR), immunology tests, and computed tomography (CT) for the emergency department (ED) diagnosis of COVID-19. Secondary objectives included a synopsis of diagnostic biases likely with current COVID-19 research as well as corresponding implications of false-negative and false-positive results for clinicians and investigators.
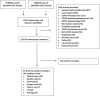
Methods: A Preferred Reporting Items for Systematic Reviews and Meta-Analyses-Scoping Review (PRISMA-ScR)-adherent synthesis of COVID-19 diagnostic accuracy through May 5, 2020, was conducted. The search strategy was designed by a medical librarian and included studies indexed by PubMed and Embase since January 2020.
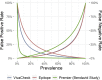
Results: A total of 1,907 citations were screened for relevance. Patients without COVID-19 are rarely reported, so specificity and likelihood ratios were generally unavailable. Fever is the most common finding, while hyposmia and hypogeusia appear useful to rule in COVID-19. Cough is not consistently present. Lymphopenia is the mostly commonly reported laboratory abnormality and occurs in over 50% of COVID-19 patients. rRT-PCR is currently considered the COVID-19 criterion standard for most diagnostic studies, but a single test sensitivity ranges from 60% to 78%. Multiple reasons for false-negatives rRT-PCR exist, including sample site tested and disease stage during which sample was obtained. CT may increase COVID-19 sensitivity in conjunction with rRT-PCR, but guidelines for imaging patients most likely to benefit are emerging. IgM and IgG serology levels are undetectable in the first week of COVID-19, but sensitivity (range = 82% to 100%) and specificity (range = 87% to 100%) are promising. Whether detectable COVID-19 antibodies correspond to immunity remains unanswered. Current studies do not adhere to accepted diagnostic accuracy reporting standards and likely report significantly biased results if the same tests were to be applied to general ED populations with suspected COVID-19.
Conclusions: With the exception of fever and disorders of smell/taste, history and physical examination findings are unhelpful to distinguish COVID-19 from other infectious conditions that mimic SARS-CoV-2 like influenza. Routine laboratory tests are also nondiagnostic, although lymphopenia is a common finding and other abnormalities may predict severe disease. Although rRT-PCR is the current criterion standard, more inclusive consensus-based criteria will likely emerge because of the high false-negative rate of PCR tests. The role of serology and CT in ED assessments remains undefined.
© 2020 by the Society for Academic Emergency Medicine.
Figures

Similar articles
-
Thoracic imaging tests for the diagnosis of COVID-19.Cochrane Database Syst Rev. 2020 Sep 30;9:CD013639. doi: 10.1002/14651858.CD013639.pub2. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2020 Nov 26;11:CD013639. doi: 10.1002/14651858.CD013639.pub3 PMID: 32997361 Updated.
-
Universal screening for SARS-CoV-2 infection: a rapid review.Cochrane Database Syst Rev. 2020 Sep 15;9(9):CD013718. doi: 10.1002/14651858.CD013718. Cochrane Database Syst Rev. 2020. PMID: 33502003 Free PMC article.
-
Rapid, point-of-care antigen tests for diagnosis of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2022 Jul 22;7(7):CD013705. doi: 10.1002/14651858.CD013705.pub3. Cochrane Database Syst Rev. 2022. PMID: 35866452 Free PMC article. Review.
-
Routine laboratory testing to determine if a patient has COVID-19.Cochrane Database Syst Rev. 2020 Nov 19;11(11):CD013787. doi: 10.1002/14651858.CD013787. Cochrane Database Syst Rev. 2020. PMID: 33211319 Free PMC article.
-
Diagnostic Accuracy of History, Physical Examination, Laboratory Tests, and Point-of-care Ultrasound for Pediatric Acute Appendicitis in the Emergency Department: A Systematic Review and Meta-analysis.Acad Emerg Med. 2017 May;24(5):523-551. doi: 10.1111/acem.13181. Acad Emerg Med. 2017. PMID: 28214369 Review.
Cited by
-
Ending the Pandemic: Are Rapid COVID-19 Tests a Step Forward or Back?West J Emerg Med. 2021 May 19;22(3):543-546. doi: 10.5811/westjem.2021.2.50550. West J Emerg Med. 2021. PMID: 34125024 Free PMC article.
-
Emergency response ability training of nursing students in the emergency department under COVID-19 epidemic situation-expert consensus of evidence-based practice and Delphi method.Am J Transl Res. 2022 Dec 15;14(12):8969-8979. eCollection 2022. Am J Transl Res. 2022. PMID: 36628245 Free PMC article.
-
Age-related differences in symptoms in older emergency department patients with COVID-19: Prevalence and outcomes in a multicenter cohort.J Am Geriatr Soc. 2022 Jul;70(7):1918-1930. doi: 10.1111/jgs.17816. Epub 2022 Apr 29. J Am Geriatr Soc. 2022. PMID: 35460268 Free PMC article.
-
False-negative real-time polymerase chain reaction tests in COVID-19 patients: an epidemiological analysis of 302 patients.Public Health. 2021 Nov;200:84-90. doi: 10.1016/j.puhe.2021.09.010. Epub 2021 Sep 22. Public Health. 2021. PMID: 34710718 Free PMC article.
-
Sort and Sieve: Pre-Triage Screening of Patients with Suspected COVID-19 in the Emergency Department.Int J Environ Res Public Health. 2021 Sep 2;18(17):9271. doi: 10.3390/ijerph18179271. Int J Environ Res Public Health. 2021. PMID: 34501861 Free PMC article.
References
-
- Lei P, Fan B, Wang P Differential diagnosis for coronavirus disease (COVID‐ 19): beyond radiologic features. AJR Am J Roentgenol 2020;215:W19. - PubMed
-
- U.S. Food and Drug Administration . Emergency Use Authorization (EUA) information, and list of all current EUAs Food and Drug Administration. 2020. Available at: https://www.fda.gov/emergency‐preparedness‐and‐response/mcm‐legal‐regula.... Accessed Jun 3, 2020.
-
- Lippi G, Plebani M The critical role of laboratory medicine during coronavirus disease 2019 (COVID‐19) and other viral outbreaks. Clin Chem Lab Med 2020;58:1063–9. - PubMed
-
- Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA‐ScR): checklist and explanation. Ann Intern Med 2018;169:476–3. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

